Neural KCNQ (Kv7) channels
- PMID: 19298256
- PMCID: PMC2697739
- DOI: 10.1111/j.1476-5381.2009.00111.x
Neural KCNQ (Kv7) channels
Abstract
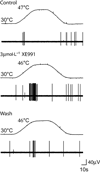
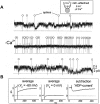
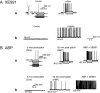
KCNQ genes encode five Kv7 K(+) channel subunits (Kv7.1-Kv7.5). Four of these (Kv7.2-Kv7.5) are expressed in the nervous system. Kv7.2 and Kv7.3 are the principal molecular components of the slow voltage-gated M-channel, which widely regulates neuronal excitability, although other subunits may contribute to M-like currents in some locations. M-channels are closed by receptors coupled to Gq such as M1 and M3 muscarinic receptors; this increases neuronal excitability and underlies some forms of cholinergic excitation. Muscarinic closure results from activation of phospholipase C and consequent hydrolysis and depletion of membrane phosphatidylinositol-4,5-bisphosphate, which is required for channel opening. Some effects of M-channel closure, determined from transmitter action, selective blocking drugs (linopirdine and XE991) and KCNQ2 gene disruption or manipulation, are as follows: (i) in sympathetic neurons: facilitation of repetitive discharges and conversion from phasic to tonic firing; (ii) in sensory nociceptive systems: facilitation of A-delta peripheral sensory fibre responses to noxious heat; and (iii) in hippocampal pyramidal neurons: facilitation of repetitive discharges, enhanced after-depolarization and burst-firing, and induction of spontaneous firing through a reduction of action potential threshold at the axon initial segment. Several drugs including flupirtine and retigabine enhance neural Kv7/M-channel activity, principally through a hyperpolarizing shift in their voltage gating. In consequence they reduce neural excitability and can inhibit nociceptive stimulation and transmission. Flupirtine is in use as a central analgesic; retigabine is under clinical trial as a broad-spectrum anticonvulsant and is an effective analgesic in animal models of chronic inflammatory and neuropathic pain.
Figures







References
-
- Adams PR, Jones SW, Pennefather P, Brown DA, Kock C, Lancaster B. Slow synaptic transmission in frog sympathetic ganglia. J Exp Biol. 1986;124:259–285. - PubMed
-
- Aiken SP, Zaczek R, Brown BS. Pharmacology of the neurotransmitter release enhancer linopirdine (DuP 996), and insights into its mechanism of action. Adv Pharmacol. 1996;35:349–384. - PubMed
-
- Bentzen BH, Schmitt N, Calloe K, Dalby Brown W, Grunnet M, Olesen SP. The acrylamide (S)-1 differentially affects Kv7 (KCNQ) potassium channels. Neuropharmacology. 2006;51:1068–1077. - PubMed
-
- Blackburn-Munro G, Jensen BS. The anticonvulsant retigabine attenuates nociceptive behaviours in rat models of persistent and neuropathic pain. Eur J Pharmacol. 2003;460:109–116. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

