Measuring the activity of BioBrick promoters using an in vivo reference standard
- PMID: 19298678
- PMCID: PMC2683166
- DOI: 10.1186/1754-1611-3-4
Measuring the activity of BioBrick promoters using an in vivo reference standard
Abstract
Background: The engineering of many-component, synthetic biological systems is being made easier by the development of collections of reusable, standard biological parts. However, the complexity of biology makes it difficult to predict the extent to which such efforts will succeed. As a first practical example, the Registry of Standard Biological Parts started at MIT now maintains and distributes thousands of BioBrick standard biological parts. However, BioBrick parts are only standardized in terms of how individual parts are physically assembled into multi-component systems, and most parts remain uncharacterized. Standardized tools, techniques, and units of measurement are needed to facilitate the characterization and reuse of parts by independent researchers across many laboratories.
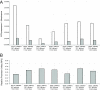
Results: We found that the absolute activity of BioBrick promoters varies across experimental conditions and measurement instruments. We choose one promoter (BBa_J23101) to serve as an in vivo reference standard for promoter activity. We demonstrated that, by measuring the activity of promoters relative to BBa_J23101, we could reduce variation in reported promoter activity due to differences in test conditions and measurement instruments by approximately 50%. We defined a Relative Promoter Unit (RPU) in order to report promoter characterization data in compatible units and developed a measurement kit so that researchers might more easily adopt RPU as a standard unit for reporting promoter activity. We distributed a set of test promoters to multiple labs and found good agreement in the reported relative activities of promoters so measured. We also characterized the relative activities of a reference collection of BioBrick promoters in order to further support adoption of RPU-based measurement standards.
Conclusion: Relative activity measurements based on an in vivoreference standard enables improved measurement of promoter activity given variation in measurement conditions and instruments. These improvements are sufficient to begin to support the measurement of promoter activities across many laboratories. Additional in vivo reference standards for other types of biological functions would seem likely to have similar utility, and could thus improve research on the design, production, and reuse of standard biological parts.
Figures
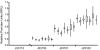
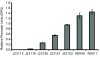
References
-
- Knight T. Idempotent Vector Design for Standard Assembly of Biobricks. 2003. http://web.mit.edu/synbio/release/docs/biobricks.pdf
-
- Arkin AP, Endy D. A Standard Parts List for Biological Circuitry. DARPA White Paper. 1999. http://hdl.handle.net/1721.1/29794
-
- Endy D. 2003 Synthetic Biology study. 2003. http://hdl.handle.net/1721.1/38455
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

