Dynamic analysis of apoptosis using cyanine SYTO probes: from classical to microfluidic cytometry
- PMID: 19298813
- PMCID: PMC3874874
- DOI: 10.1016/j.yexcr.2009.03.006
Dynamic analysis of apoptosis using cyanine SYTO probes: from classical to microfluidic cytometry
Abstract
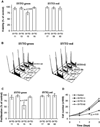
Cell death is a stochastic process, often initiated and/or executed in a multi-pathway/multi-organelle fashion. Therefore, high-throughput single-cell analysis platforms are required to provide detailed characterization of kinetics and mechanisms of cell death in heterogeneous cell populations. However, there is still a largely unmet need for inert fluorescent probes, suitable for prolonged kinetic studies. Here, we compare the use of innovative adaptation of unsymmetrical SYTO dyes for dynamic real-time analysis of apoptosis in conventional as well as microfluidic chip-based systems. We show that cyanine SYTO probes allow non-invasive tracking of intracellular events over extended time. Easy handling and "stain-no wash" protocols open up new opportunities for high-throughput analysis and live-cell sorting. Furthermore, SYTO probes are easily adaptable for detection of cell death using automated microfluidic chip-based cytometry. Overall, the combined use of SYTO probes and state-of-the-art Lab-on-a-Chip platform emerges as a cost effective solution for automated drug screening compared to conventional Annexin V or TUNEL assays. In particular, it should allow for dynamic analysis of samples where low cell number has so far been an obstacle, e.g. primary cancer stems cells or circulating minimal residual tumors.
Figures
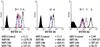



References
-
- Wlodkowic D, Skommer J, Darzynkiewicz Z. SYTO probes in cytometry of tumor cell death. Cytometry A. 2008;73(6):496–507. - PubMed
-
- Martin RM, Leonhardt H, Cardoso MC. DNA labeling in living cells. Cytometry A. 2005;67A:45–52. - PubMed
-
- Wlodkowic D, Skommer J, Pelkonen J. Towards an understanding of apoptosis detection by SYTO dyes. Cytometry A. 2007;71(2):61–72. - PubMed
-
- Wlodkowic D, Skommer J, Hillier Z. Darzynkiewicz, Multiparameter detection of apoptosis using red-excitable SYTO probes. Cytometry A. 2008;73(6):563–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

