Nucleotide- and activator-dependent structural and dynamic changes of arp2/3 complex monitored by hydrogen/deuterium exchange and mass spectrometry
- PMID: 19298826
- PMCID: PMC2983480
- DOI: 10.1016/j.jmb.2009.03.028
Nucleotide- and activator-dependent structural and dynamic changes of arp2/3 complex monitored by hydrogen/deuterium exchange and mass spectrometry
Abstract
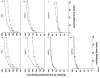
Arp2/3 complex plays a central role in the de novo nucleation of filamentous actin as branches on existing filaments. The complex must bind ATP, protein activators [e.g., Wiskott-Aldrich syndrome protein (WASp)], and the side of an actin filament to form a new actin filament. Amide hydrogen/deuterium exchange coupled with mass spectrometry was used to examine the structural and dynamic properties of the mammalian Arp2/3 complex in the presence of both ATP and the activating peptide segment from WASp. Changes in the rate of hydrogen exchange indicate that ATP binding causes conformational rearrangements of Arp2 and Arp3 that are transmitted allosterically to the Arp complex (ARPC)1, ARPC2, ARPC4, and ARPC5 subunits. These data are consistent with the closure of nucleotide-binding cleft of Arp3 upon ATP binding, resulting in structural rearrangements that propagate throughout the complex. Binding of the VCA domain of WASp to ATP-Arp2/3 further modulates the rates of hydrogen exchange in these subunits, indicating that a global conformational reorganization is occurring. These effects may include the direct binding of activators to Arp3, Arp2, and ARPC1; alterations in the relative orientations of Arp2 and Arp3; and the long-range transmission of activator-dependent signals to segments proposed to be involved in binding the F-actin mother filament.
Figures


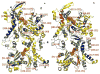
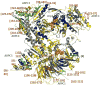
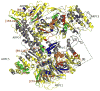
References
-
- Pollard TD, Earnshaw WC. Cell Biology. Saunders; Philadelphia: 2004. updated edition edit.
-
- Gournier H, Goley ED, Niederstrasser H, Trinh T, Welch MD. Reconstitution of human Arp2/3 complex reveals critical roles of individual subunits in complex structure and activity. Mol Cell. 2001;8:1041–52. - PubMed
-
- Machesky LM, Gould KL. The Arp2/3 complex: a multifunctional actin organizer. Curr Opin Cell Biol. 1999;11:117–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

