Control of viremia and maintenance of intestinal CD4(+) memory T cells in SHIV(162P3) infected macaques after pathogenic SIV(MAC251) challenge
- PMID: 19298994
- PMCID: PMC2674129
- DOI: 10.1016/j.virol.2009.02.014
Control of viremia and maintenance of intestinal CD4(+) memory T cells in SHIV(162P3) infected macaques after pathogenic SIV(MAC251) challenge
Abstract
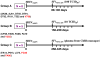
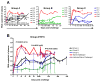
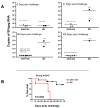
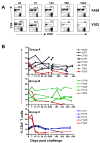
Recent HIV vaccine failures have prompted calls for more preclinical vaccine testing in non-human primates. However, similar to HIV infection of humans, developing a vaccine that protects macaques from infection following pathogenic SIV(MAC251) challenge has proven difficult, and current vaccine candidates at best, only reduce viral loads after infection. Here we demonstrate that prior infection with a chimeric simian-human immunodeficiency virus (SHIV) containing an HIV envelope gene confers protection against intravenous infection with the heterologous, highly pathogenic SIV(MAC251) in rhesus macaques. Although definitive immune correlates of protection were not identified, preservation and/or restoration of intestinal CD4(+) memory T cells were associated with protection from challenge and control of viremia. These results suggest that protection against pathogenic lentiviral infection or disease progression is indeed possible, and may correlate with preservation of mucosal CD4(+) T cells.
Figures


References
-
- Abel K, Compton L, Rourke T, Montefiori D, Lu D, Rothaeusler K, Fritts L, Bost K, Miller CJ. Simian-human immunodeficiency virus SHIV89.6-induced protection against intravaginal challenge with pathogenic SIVmac239 is independent of the route of immunization and is associated with a combination of cytotoxic T-lymphocyte and alpha interferon responses. J Virol. 2003;77(5):3099–118. - PMC - PubMed
-
- Altfeld M, Allen TM, Yu XG, Johnston MN, Agrawal D, Korber BT, Montefiori DC, O’Connor DH, Davis BT, Lee PK, Maier EL, Harlow J, Goulder PJ, Brander C, Rosenberg ES, Walker BD. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature. 2002;420(6914):434–9. - PubMed
-
- Casimiro DR, Wang F, Schleif WA, Liang X, Zhang ZQ, Tobery TW, Davies ME, McDermott AB, O’Connor DH, Fridman A, Bagchi A, Tussey LG, Bett AJ, Finnefrock AC, Fu TM, Tang A, Wilson KA, Chen M, Perry HC, Heidecker GJ, Freed DC, Carella A, Punt KS, Sykes KJ, Huang L, Ausensi VI, Bachinsky M, Sadasivan-Nair U, Watkins DI, Emini EA, Shiver JW. Attenuation of simian immunodeficiency virus SIVmac239 infection by prophylactic immunization with dna and recombinant adenoviral vaccine vectors expressing Gag. J Virol. 2005;79(24):15547–55. - PMC - PubMed
-
- Cline AN, Bess JW, Piatak M, Jr, Lifson JD. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34(5–6):303–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

