The LDL receptor
- PMID: 19299327
- PMCID: PMC2740366
- DOI: 10.1161/ATVBAHA.108.179564
The LDL receptor
Abstract
In this article, the history of the LDL receptor is recounted by its codiscoverers. Their early work on the LDL receptor explained a genetic cause of heart attacks and led to new ways of thinking about cholesterol metabolism. The LDL receptor discovery also introduced three general concepts to cell biology: receptor-mediated endocytosis, receptor recycling, and feedback regulation of receptors. The latter concept provides the mechanism by which statins selectively lower plasma LDL, reducing heart attacks and prolonging life.
Figures



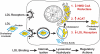
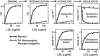

References
-
- Muller C. Xanthomata, hypercholesterolemia, angina pectoris. Acta Med Scand. 1938;89:75–84.
-
- Khachadurian AK. The inheritance of essential familial hypercholesterolemia. Am J Med. 1964;37:402–7. - PubMed
-
- Goldstein JL, Hobbs HH, Brown MS. Familial hypercholesterolemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th ed. McGraw-Hill, Inc.; New York: 2001. pp. 2863–913.
-
- Bailey JM. Regulation of cell cholesterol content. In: Porter R, Knight J, editors. Atherogenesis: Initiating Factors. 12 ed. Ciba Foundation Symposium; Elsevier, Amsterdam: 1973. pp. 63–92.
-
- Rothblat GH. Lipid metabolism in tissue culture cells. Adv Lipid Res. 1969;7:l35–l62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

