How we treat cytomegalovirus in hematopoietic cell transplant recipients
- PMID: 19299333
- PMCID: PMC2700312
- DOI: 10.1182/blood-2008-10-143560
How we treat cytomegalovirus in hematopoietic cell transplant recipients
Abstract
Cytomegalovirus (CMV) continues to cause major complications after hematopoietic cell transplantation (HCT). Over the past decade, most centers have adopted preemptive antiviral treatment or prophylaxis strategies to prevent CMV disease. Both strategies are effective but also have shortcomings with presently available drugs. Here, we review aspects of CMV treatment and prevention in HCT recipients, including currently used drugs and diagnostics, ways to optimize preemptive therapy strategies with quantitative polymerase chain reaction assays, the use of prophylaxis, management of CMV disease caused by wild-type or drug-resistant strains, and future strategies.
Figures

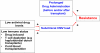

References
-
- Boeckh M, Leisenring W, Riddell SR, et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–414. - PubMed
-
- Krause H, Hebart H, Jahn G, Muller CA, Einsele H. Screening for CMV-specific T cell proliferation to identify patients at risk of developing late onset CMV disease. Bone Marrow Transplant. 1997;19:1111–1116. - PubMed
-
- Zaia JA, Gallez-Hawkins GM, Tegtmeier BR, et al. Late cytomegalovirus disease in marrow transplantation is predicted by virus load in plasma. J Infect Dis. 1997;176:782–785. - PubMed
-
- Broers AE, van Der Holt R, van Esser JW, et al. Increased transplant-related morbidity and mortality in CMV-seropositive patients despite highly effective prevention of CMV disease after allogeneic T-cell-depleted stem cell transplantation. Blood. 2000;95:2240–2245. - PubMed
-
- Craddock C, Szydlo RM, Dazzi F, et al. Cytomegalovirus seropositivity adversely influences outcome after T-depleted unrelated donor transplant in patients with chronic myeloid leukaemia: the case for tailored graft-versus-host disease prophylaxis. Br J Haematol. 2001;112:228–236. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

