Reciprocal responsiveness to interleukin-12 and interferon-alpha specifies human CD8+ effector versus central memory T-cell fates
- PMID: 19299334
- PMCID: PMC2689051
- DOI: 10.1182/blood-2008-11-188458
Reciprocal responsiveness to interleukin-12 and interferon-alpha specifies human CD8+ effector versus central memory T-cell fates
Abstract
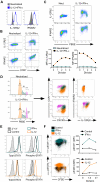
Multiple innate signals regulate the genesis of effector and memory CD8+ T cells. In this study, we demonstrate that the innate cytokines interleukin (IL)-12 and interferon (IFN)-alpha/beta regulate distinct aspects of effector and memory human CD8+ T-cell differentiation. IL-12 exclusively promoted the development of IFN-gamma- and tumor necrosis factor (TNF)-alpha-secreting T effector memory (T(EM)) cells, whereas IFN-alpha drove the development of T central memory (T(CM)) cells. The development of T(EM) and T(CM) was linked to cell division. In rapidly dividing cells, IL-12 programmed T(EM) through induction of the IL-12 receptor beta2. In contrast, IFN-alpha regulated T(CM) development by slowing the progression of cell division in a subpopulation of cells that selectively expressed elevated IFN-alpha/beta receptor-2. The strength of signal delivered through T-cell receptor (TCR) engagement regulated the responsiveness of cells to IL-12 and IFN-alpha. In the presence of both IL-12 and IFN-alpha, these cytokine signals were amplified as the strength of the TCR signal was increased, promoting the simultaneous development of both T(CM) and T(EM). Together, our results support a novel model in which IL-12 and IFN-alpha act in a nonredundant manner to regulate the colinear generation of both effector and memory cells.
Figures
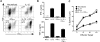





References
-
- Mescher MF, Curtsinger JM, Agarwal P, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. - PubMed
-
- Valenzuela J, Schmidt C, Mescher M. The roles of IL-12 in providing a third signal for clonal expansion of naive CD8 T cells. J Immunol. 2002;169:6842–6849. - PubMed
-
- Curtsinger JM, Valenzuela JO, Agarwal P, Lins D, Mescher MF. Type I IFNs provide a third signal to CD8 T cells to stimulate clonal expansion and differentiation. J Immunol. 2005;174:4465–4469. - PubMed
-
- Casey KA, Mescher MF. IL-21 promotes differentiation of naive CD8 T cells to a unique effector phenotype. J Immunol. 2007;178:7640–7648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

