Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis
- PMID: 19299462
- PMCID: PMC2714439
- DOI: 10.1242/jcs.040717
Low-density lipoprotein receptor-related protein 1 is an essential receptor for myelin phagocytosis
Abstract
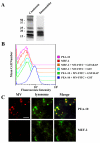
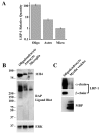
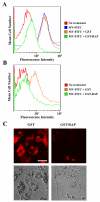
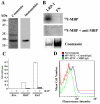
Multiple sclerosis (MS) is an autoimmune disease in which myelin is progressively degraded. Because degraded myelin may both initiate and accelerate disease progression, clearing degraded myelin from extracellular spaces may be critical. In this study, we prepared myelin vesicles (MV) from rat brains as a model of degraded myelin. Murine embryonic fibroblasts (MEFs) rapidly internalized MVs, which accumulated in lysosomes only when these cells expressed low-density lipoprotein receptor-related protein (LRP1). Receptor-associated protein (RAP), which binds LRP1 and inhibits interaction with other ligands, blocked MV uptake by LRP1-expressing MEFs. As a complementary approach, we prepared primary cultures of rat astrocytes, microglia and oligodendrocytes. All three cell types expressed LRP1 and mediated MV uptake, which was inhibited by RAP. LRP1 gene-silencing in oligodendrocytes also blocked MV uptake. Myelin basic protein (MBP), which was expressed as a recombinant protein, bound directly to LRP1. MBP-specific antibody inhibited MV uptake by oligodendrocytes. In experimental autoimmune encephalomyelitis in mice, LRP1 protein expression was substantially increased in the cerebellum and spinal cord. LRP1 colocalized with multiple CNS cell types. These studies establish LRP1 as a major receptor for phagocytosis of degraded myelin, which may function alone or in concert with co-receptors previously implicated in myelin phagocytosis.
Figures

Similar articles
-
Identification of the low density lipoprotein (LDL) receptor-related protein-1 interactome in central nervous system myelin suggests a role in the clearance of necrotic cell debris.J Biol Chem. 2013 Feb 15;288(7):4538-48. doi: 10.1074/jbc.M112.384693. Epub 2012 Dec 21. J Biol Chem. 2013. PMID: 23264627 Free PMC article.
-
LRP1 expression in microglia is protective during CNS autoimmunity.Acta Neuropathol Commun. 2016 Jul 11;4(1):68. doi: 10.1186/s40478-016-0343-2. Acta Neuropathol Commun. 2016. PMID: 27400748 Free PMC article.
-
LDL receptor-related protein-1 is a sialic-acid-independent receptor for myelin-associated glycoprotein that functions in neurite outgrowth inhibition by MAG and CNS myelin.J Cell Sci. 2013 Jan 1;126(Pt 1):209-20. doi: 10.1242/jcs.113191. Epub 2012 Nov 6. J Cell Sci. 2013. PMID: 23132925 Free PMC article.
-
LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies.Physiol Rev. 2008 Jul;88(3):887-918. doi: 10.1152/physrev.00033.2007. Physiol Rev. 2008. PMID: 18626063 Free PMC article. Review.
-
LDL receptor-related protein 1 and its interacting partners in tissue homeostasis.Curr Opin Lipidol. 2021 Oct 1;32(5):301-307. doi: 10.1097/MOL.0000000000000776. Curr Opin Lipidol. 2021. PMID: 34310383 Free PMC article. Review.
Cited by
-
The Dual Role of Low-Density Lipoprotein Receptor-Related Protein 1 in Atherosclerosis.Front Cardiovasc Med. 2021 May 28;8:682389. doi: 10.3389/fcvm.2021.682389. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34124208 Free PMC article. Review.
-
Citrullinated isomer of myelin basic protein can induce inflammatory responses in astrocytes.IBRO Neurosci Rep. 2023 Dec 19;16:127-134. doi: 10.1016/j.ibneur.2023.12.003. eCollection 2024 Jun. IBRO Neurosci Rep. 2023. PMID: 38288135 Free PMC article.
-
Unlocking the Potential: immune functions of oligodendrocyte precursor cells.Front Immunol. 2024 Jul 9;15:1425706. doi: 10.3389/fimmu.2024.1425706. eCollection 2024. Front Immunol. 2024. PMID: 39044821 Free PMC article. Review.
-
Mechanisms by Which LRP1 (Low-Density Lipoprotein Receptor-Related Protein-1) Maintains Arterial Integrity.Arterioscler Thromb Vasc Biol. 2018 Nov;38(11):2548-2549. doi: 10.1161/ATVBAHA.118.311882. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354248 Free PMC article. No abstract available.
-
Low-Density Lipoprotein Receptor Deficiency Attenuates Neuroinflammation through the Induction of Apolipoprotein E.Front Immunol. 2017 Nov 30;8:1701. doi: 10.3389/fimmu.2017.01701. eCollection 2017. Front Immunol. 2017. PMID: 29276512 Free PMC article.
References
-
- Adams, R. A., Bauer, J., Flick, M. J., Sikorski, S. L., Nuriel, T., Lassmann, H., Degen, J. L. and Akassoglou, K. (2007). The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J. Exp. Med. 204, 571-582. - PMC - PubMed
-
- Antony, J. M., van Marle, G., Opii, W., Butterfield, D. A., Mallet, F., Yong, V. W., Wallace, J. L., Deacon, R. M., Warren, K. and Power, C. (2004). Human endogenous retrovirus glycoprotein-mediated induction of redox reactants causes oligodendrocyte death and demyelination. Nat. Neurosci. 7, 1088-1095. - PubMed
-
- Arnold-Schild, D., Hanau, D., Spehner, D., Schmid, C., Rammensee, H. G., de la Salle, H. and Schild, H. (1999). Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J. Immunol. 162, 3757-3760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

