Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis
- PMID: 19299512
- PMCID: PMC2682911
- DOI: 10.1074/jbc.M807598200
Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis
Abstract
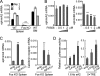
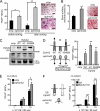
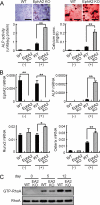
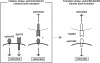
Bone is remodeled constantly throughout life by bone-resorbing osteoclasts and bone-forming osteoblasts. To maintain bone volume and quality, differentiation of osteoclasts and osteoblasts is tightly regulated through communication between and within these two cell lineages. Previously we reported that cell-cell interaction mediated by ephrinB2 ligand on osteoclasts and EphB4 receptor on osteoblasts generates bidirectional anti-osteoclastogenic and pro-osteoblastogenic signals into respective cells and presumably facilitates transition from bone resorption to bone formation. Here we show that bidirectional ephrinA2-EphA2 signaling regulates bone remodeling at the initiation phase. EphrinA2 expression was rapidly induced by receptor activator of NF-kappaB ligand in osteoclast precursors; this was dependent on the transcription factor c-Fos but independent of the c-Fos target gene product NFATc1. Receptor EphA2 was expressed in osteoclast precursors and osteoblasts. Overexpression experiments revealed that both ephrinA2 and EphA2 in osteoclast precursors enhanced differentiation of multinucleated osteoclasts and that phospholipase Cgamma2 may mediate ephrinA2 reverse signaling. Moreover, ephrinA2 on osteoclasts was cleaved by metalloproteinases, and ephrinA2 released in the culture medium enhanced osteoclastogenesis. Interestingly, differentiation of osteoblasts lacking EphA2 was enhanced along with alkaline phosphatase, Runx2, and Osterix expression, indicating that EphA2 on osteoblasts generates anti-osteoblastogenic signals presumably by up-regulating RhoA activity. Therefore, ephrinA2-EphA2 interaction facilitates the initiation phase of bone remodeling by enhancing osteoclast differentiation and suppressing osteoblast differentiation.
Figures


References
-
- Karsenty, G., and Wagner, E. F. (2002) Dev. Cell 2 389-406 - PubMed
-
- Teitelbaum, S. L., and Ross, F. P. (2003) Nat. Rev. Genet 4 638-649 - PubMed
-
- Matsuo, K., and Irie, N. (2008) Arch. Biochem. Biophys. 473 201-209 - PubMed
-
- Grigoriadis, A. E., Wang, Z. Q., Cecchini, M. G., Hofstetter, W., Felix, R., Fleisch, H. A., and Wagner, E. F. (1994) Science 266 443-448 - PubMed
-
- Takayanagi, H., Kim, S., Koga, T., Nishina, H., Isshiki, M., Yoshida, H., Saiura, A., Isobe, M., Yokochi, T., Inoue, J., Wagner, E. F., Mak, T. W., Kodama, T., and Taniguchi, T. (2002) Dev. Cell 3 889-901 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

