Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury
- PMID: 19299628
- PMCID: PMC2674528
- DOI: 10.1161/STROKEAHA.108.540765
Methazolamide and melatonin inhibit mitochondrial cytochrome C release and are neuroprotective in experimental models of ischemic injury
Abstract
Background and purpose: The identification of a neuroprotective drug for stroke remains elusive. Given that mitochondria play a key role both in maintaining cellular energetic homeostasis and in triggering the activation of cell death pathways, we evaluated the efficacy of newly identified inhibitors of cytochrome c release in hypoxia/ischemia induced cell death. We demonstrate that methazolamide and melatonin are protective in cellular and in vivo models of neuronal hypoxia.
Methods: The effects of methazolamide and melatonin were tested in oxygen/glucose deprivation-induced death of primary cerebrocortical neurons. Mitochondrial membrane potential, release of apoptogenic mitochondrial factors, pro-IL-1beta processing, and activation of caspase -1 and -3 were evaluated. Methazolamide and melatonin were also studied in a middle cerebral artery occlusion mouse model. Infarct volume, neurological function, and biochemical events were examined in the absence or presence of the 2 drugs.
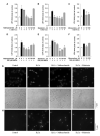
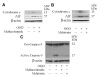
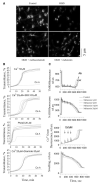
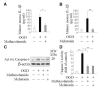
Results: Methazolamide and melatonin inhibit oxygen/glucose deprivation-induced cell death, loss of mitochondrial membrane potential, release of mitochondrial factors, pro-IL-1beta processing, and activation of caspase-1 and -3 in primary cerebrocortical neurons. Furthermore, they decrease infarct size and improve neurological scores after middle cerebral artery occlusion in mice.
Conclusions: We demonstrate that methazolamide and melatonin are neuroprotective against cerebral ischemia and provide evidence of the effectiveness of a mitochondrial-based drug screen in identifying neuroprotective drugs. Given the proven human safety of melatonin and methazolamide, and their ability to cross the blood-brain-barrier, these drugs are attractive as potential novel therapies for ischemic injury.
Figures

References
-
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, Wang X. Cytochrome c and datp-dependent formation of apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. - PubMed
-
- Chan PH. Mitochondria and neuronal death/survival signaling pathways in cerebral ischemia. Neurochem Res. 2004;29:1943–1949. - PubMed
-
- Namura S, Nagata I, Takami S, Masayasu H, Kikuchi H. Ebselen reduces cytochrome c release from mitochondria and subsequent DNA fragmen-tation after transient focal cerebral ischemia in mice. Stroke. 2001;32:1906–1911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

