CLEC-2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils
- PMID: 19299712
- PMCID: PMC2727695
- DOI: 10.4049/jimmunol.0802808
CLEC-2 is a phagocytic activation receptor expressed on murine peripheral blood neutrophils
Abstract
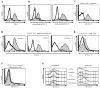
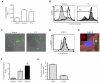
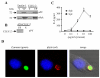
CLEC-2 is a member of the "dectin-1 cluster" of C-type lectin-like receptors and was originally thought to be restricted to platelets. In this study, we demonstrate that murine CLEC-2 is also expressed by peripheral blood neutrophils, but only weakly by bone marrow or elicited inflammatory neutrophils. On circulating neutrophils, CLEC-2 can mediate phagocytosis of Ab-coated beads and the production of proinflammatory cytokines, including TNF-alpha, in response to the CLEC-2 ligand, rhodocytin. CLEC-2 possesses a tyrosine-based cytoplasmic motif similar to that of dectin-1, and we show using chimeric analyses that the activities of this receptor are dependent on this tyrosine. Like dectin-1, CLEC-2 can recruit the signaling kinase Syk in myeloid cells, however, stimulation of this pathway does not induce the respiratory burst. These data therefore demonstrate that CLEC-2 expression is not restricted to platelets and that it functions as an activation receptor on neutrophils.
Figures


References
-
- Drickamer K, Fadden AJ. Genomic analysis of C-type lectins. Biochemical Society symposium. 2002:59–72. - PubMed
-
- Zelensky AN, Gready JE. The C-type lectin-like domain superfamily. The FEBS journal. 2005;272:6179–6217. - PubMed
-
- Sobanov Y, Bernreiter A, Derdak S, Mechtcheriakova D, Schweighofer B, Duchler M, Kalthoff F, Hofer E. A novel cluster of lectin-like receptor genes expressed in monocytic, dendritic and endothelial cells maps close to the NK receptor genes in the human NK gene complex. Eur. J. Immunol. 2001;31:3493–3503. - PubMed
-
- Pyz E, Marshall AS, Gordon S, Brown GD. C-type lectin-like receptors on myeloid cells. Annals of medicine. 2006;38:242–251. - PubMed
-
- Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat. Rev. Immunol. 2003;3:304–316. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

