The cyclic AMP response element modulator {alpha} suppresses CD86 expression and APC function
- PMID: 19299714
- PMCID: PMC2786066
- DOI: 10.4049/jimmunol.0802976
The cyclic AMP response element modulator {alpha} suppresses CD86 expression and APC function
Erratum in
- J Immunol. 2009 Jul 1;183(1):770
Abstract
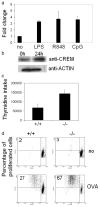
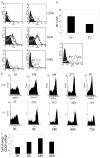
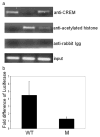
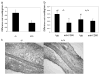
The cAMP response element modulator (CREM)alpha is a widely expressed transcriptional repressor that is important for the termination of the T cell immune response and contributes to the abnormal T cell function in patients with systemic lupus erythematosus. We present evidence that APCs of Crem(-/-) mice express increased amounts of the costimulatory molecule CD86 and induce enhanced Ag-dependent and Ag-independent T cell proliferation. Similarly, human APCs in which CREMalpha was selectively suppressed expressed more CD86 on the surface membrane. CREMalpha was found to bind to the CD86 promoter and suppressed its activity. Transfer of APCs from Crem(-/-) mice into naive mice facilitated a significantly stronger contact dermatitis response compared with mice into which APCs from Crem(+/+) mice had been transferred. We conclude that CREMalpha is an important negative regulator of costimulation and APC-dependent T cell function both in vitro and in vivo.
Conflict of interest statement
The authors have no financial conflict of interest.
Figures

References
-
- Powell JD, Lerner CG, Ewoldt GR, Schwartz RH. The −180 site of the IL-2 promoter is the target of CREB/CREM binding in T cell anergy. J Immunol. 1999;163:6631–6639. - PubMed
-
- Tenbrock K, Juang YT, Tolnay M, Tsokos GC. The cyclic adenosine 5′-monophosphate response element modulator suppresses IL-2 production in stimulated T cells by a chromatin-dependent mechanism. J Immunol. 2003;170:2971–2976. - PubMed
-
- Tenbrock K, Juang YT, Leukert N, Roth J, Tsokos GC. The transcriptional repressor cAMP response element modulator alpha interacts with histone deacetylase 1 to repress promoter activity. J Immunol. 2006;177:6159–6164. - PubMed
-
- Tenbrock K, Juang YT, Gourley MF, Nambiar MP, Tsokos GC. Antisense cyclic adenosine 5′-monophosphate response element modulator up-regulates IL-2 in T cells from patients with systemic lupus erythematosus. J Immunol. 2002;169:4147–4152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

