Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1
- PMID: 19299723
- PMCID: PMC2713929
- DOI: 10.4049/jimmunol.0802041
Recall responses by helpless memory CD8+ T cells are restricted by the up-regulation of PD-1
Abstract
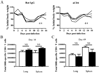
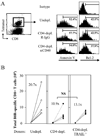
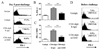
CD4 help is crucial for memory CD8(+) T cell development, yet the mechanisms of CD4 help and why (CD4) helpless memory CD8(+) T cells elicit poor recall responses are currently not well understood. In this study we investigated these questions using an in vivo acute virus infection model. We show herein that CD4 help during priming is required for memory CD8(+) T cell differentiation, and that stimulation of CD40 during priming rescues the helpless defects in the absence of CD4(+) T cells. The defective recall response by helpless memory cells did not correlate with the amount of cell death and was independent of TRAIL. However, helpless memory cells excessively up-regulated the inhibitory receptor PD-1 (programmed cell death-1), and PD-1 blockade enhanced the recall response of helpless memory cells. Furthermore, providing IL-2 signaling in vivo during the recall response reduced PD-1 expression and rescued the recall response of helpless memory cells. Our study identifies molecular pathways involved in CD4 help for memory CD8(+) T cell generation that are independent of TRAIL, and it provides therapeutic implications that helpless memory cell function can be restored at multiple stages through various immunological interventions.
Figures







References
-
- Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. - PubMed
-
- Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. - PubMed
-
- Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. - PubMed
-
- Khanolkar A, Fuller MJ, Zajac AJ. CD4 T cell-dependent CD8 T cell maturation. J Immunol. 2004;172:2834–2844. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

