Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment
- PMID: 19299740
- PMCID: PMC2749517
- DOI: 10.4049/jimmunol.0803659
Isoforms of vitamin E have opposing immunoregulatory functions during inflammation by regulating leukocyte recruitment
Erratum in
- J Immunol. 2010 Jul 15;185(2):1341
Abstract
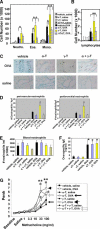
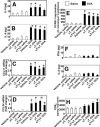
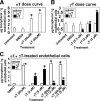
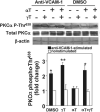
Reports indicate contradictory outcomes for anti-inflammatory functions of the alpha-tocopherol isoform of vitamin E in clinical studies of asthma and atherosclerosis. These seemingly disparate clinical results are consistent with novel unrecognized properties of isoforms of vitamin E reported in this study. We demonstrate that the isoform d-gamma-tocopherol elevates inflammation in experimental asthma. Moreover, d-gamma-tocopherol, at as little as 10% the concentration of d-alpha-tocopherol, ablates the anti-inflammatory benefit of the d-alpha-tocopherol isoform. A mechanism for these opposing immunoregulatory functions of purified tocopherols at physiological concentrations is not through modulation of expression of several cytokines, chemokines, or adhesion molecules, but is, at least in part, by regulation of endothelial cell signals during leukocyte recruitment. These opposing regulatory functions of vitamin E isoforms have impact on interpretations of vitamin E studies. In summary, our studies with purified tocopherol isoforms alter our understanding of vitamin E regulation of vascular function and asthma.
Figures




References
-
- Kalayci O, Besler T, Kilinc K, Sekerel BE, Saraclar Y. Serum levels of antioxidant vitamins (α tocopherol, β carotene, and ascorbic acid) in children with bronchial asthma. Turk. J. Peds. 2000;42:17–21. - PubMed

