MRI assessment of ischemic liver after intraportal islet transplantation
- PMID: 19300184
- PMCID: PMC2779521
- DOI: 10.1097/TP.0b013e318199c7d2
MRI assessment of ischemic liver after intraportal islet transplantation
Abstract
Background: There is a recent focus on embolization of the portal vein by transplanted islets as a major cause of early graft loss. The resultant ischemia causes necrosis or apoptosis of cells within the liver. Thus, noninvasive assessment of the liver receiving the islet transplant is important to evaluate the status islet grafts.
Methods: This study used noninvasive magnetic resonance imaging (MRI) for assessment of the posttransplant ischemic liver. Syngeneic islets in streprozotocin-induced diabetic mice were used. MRI and morphological liver assessments were performed at 0, 2, and 28 days after transplantation. Histologic assessment of insulin, hypoxia induced factor 1-alpha, and apoptosis were undertaken at similar time points.
Results: Ischemic/necrotic regions in the liver were detected by MRI at 2 days but not at 28 days after transplantation and were confirmed histologically. Liver injury was quantified from high intensity areas on T2-weighted images. Insulin release peaked 2 days after transplantation.
Conclusion: Onset and reversal of liver ischemia due to intraportal islet transplantation are detectable using T2-weighted MRI. These changes coincide with periods of maximum insulin release likely due to partial islet destruction. We propose that MRI, as a noninvasive monitor of graft-related ischemia, may be useful in assessment of liver and islet engraftment after intraportal islet transplantation in a clinical setting.
Figures

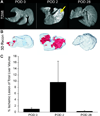
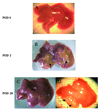


References
-
- Shapiro AM, Lakey JR, Ryan EA. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230. - PubMed
-
- Newsletter No.9. Giessen: Internationa Islet Transplnat Registry; 2001.
-
- Ryan EA, Paty BW, Senior PA. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54:2060. - PubMed
-
- Shapiro AM, Nanji SA, Lakey JR. Clinical islet transplant: current and future directions towards tolerance. Immunol Rev. 2003;196:219. - PubMed
-
- Yin D, Ding JW, Shen J, Ma L, Hara M, Chong AS. Liver ischemia contributes to early islet failure following intraportal transplantation: benefits of liver ischemic-preconditioning. Am J Transplant. 2006;6:60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

