Acid sphingomyelinase activity triggers microparticle release from glial cells
- PMID: 19300439
- PMCID: PMC2664656
- DOI: 10.1038/emboj.2009.45
Acid sphingomyelinase activity triggers microparticle release from glial cells
Erratum in
- EMBO J. 2009 May 6;28(9):1374
Abstract
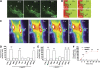
We have earlier shown that microglia, the immune cells of the CNS, release microparticles from cell plasma membrane after ATP stimulation. These vesicles contain and release IL-1beta, a crucial cytokine in CNS inflammatory events. In this study, we show that microparticles are also released by astrocytes and we get insights into the mechanism of their shedding. We show that, on activation of the ATP receptor P2X7, microparticle shedding is associated with rapid activation of acid sphingomyelinase, which moves to plasma membrane outer leaflet. ATP-induced shedding and IL-1beta release are markedly reduced by the inhibition of acid sphingomyelinase, and completely blocked in glial cultures from acid sphingomyelinase knockout mice. We also show that p38 MAPK cascade is relevant for the whole process, as specific kinase inhibitors strongly reduce acid sphingomyelinase activation, microparticle shedding and IL-1beta release. Our results represent the first demonstration that activation of acid sphingomyelinase is necessary and sufficient for microparticle release from glial cells and define key molecular effectors of microparticle formation and IL-1beta release, thus, opening new strategies for the treatment of neuroinflammatory diseases.
Figures




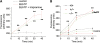
References
-
- Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C (2005) Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol 174: 7268–7277 - PubMed
-
- Brough D, Le Feuvre RA, Wheeler RD, Solovyova N, Hilfiker S, Rothwell NJ, Verkhratsky A (2003) Ca2+ stores and Ca2+ entry differentially contribute to the release of IL-1 beta and IL-1 alpha from murine macrophages. J Immunol 170: 3029–3036 - PubMed
-
- Brough D, Rothwell NJ (2007) Caspase-1-dependent processing of pro-interleukin-1beta is cytosolic and precedes cell death. J Cell Sci 120: 772–781 - PubMed
-
- Calegari F, Coco S, Taverna E, Bassetti M, Verderio C, Corradi N, Matteoli M, Rosa P (1999) A regulated secretory pathway in cultured hippocampal astrocytes. J Biol Chem 274: 22539–22547 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

