Transient cell-cell interactions in neural circuit formation
- PMID: 19300445
- PMCID: PMC3083859
- DOI: 10.1038/nrn2594
Transient cell-cell interactions in neural circuit formation
Abstract
The wiring of the nervous system requires a complex orchestration of developmental events. Emerging evidence suggests that transient cell-cell interactions often serve as positional cues for axon guidance and synaptogenesis during the assembly of neural circuits. In contrast to the relatively stable cellular interactions between synaptic partners in mature circuits, these transient interactions involve cells that are not destined to be pre- or postsynaptic cells. Here we review the roles of these transient cell-cell interactions in a variety of developmental contexts and describe the mechanisms through which they organize neural connections.
Figures

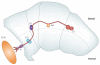

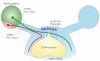

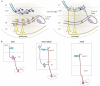

References
-
- Bate CM. Pioneer neurones in an insect embryo. Nature. 1976;260:54–56. - PubMed
-
- Bentley D, Keshishian H. Pathfinding by peripheral pioneer neurons in grasshoppers. Science. 1982;218:1082–1088. - PubMed
-
-
Bentley D, Caudy M. Pioneer axons lose directed growth after selective killing of guidepost cells. Nature. 1983;304:62–65. The first demonstration that guidepost cells play a part in axon guidance.
-
-
- Klambt C, Jacobs JR, Goodman CS. The midline of the Drosophila central nervous system: a model for the genetic analysis of cell fate, cell migration, and growth cone guidance. Cell. 1991;64:801–815. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

