Independent S-locus mutations caused self-fertility in Arabidopsis thaliana
- PMID: 19300485
- PMCID: PMC2650789
- DOI: 10.1371/journal.pgen.1000426
Independent S-locus mutations caused self-fertility in Arabidopsis thaliana
Abstract
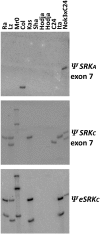
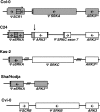
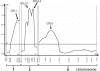
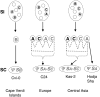
A common yet poorly understood evolutionary transition among flowering plants is a switch from outbreeding to an inbreeding mode of mating. The model plant Arabidopsis thaliana evolved to an inbreeding state through the loss of self-incompatibility, a pollen-rejection system in which pollen recognition by the stigma is determined by tightly linked and co-evolving alleles of the S-locus receptor kinase (SRK) and its S-locus cysteine-rich ligand (SCR). Transformation of A. thaliana, with a functional AlSRKb-SCRb gene pair from its outcrossing relative A. lyrata, demonstrated that A. thaliana accessions harbor different sets of cryptic self-fertility-promoting mutations, not only in S-locus genes, but also in other loci required for self-incompatibility. However, it is still not known how many times and in what manner the switch to self-fertility occurred in the A. thaliana lineage. Here, we report on our identification of four accessions that are reverted to full self-incompatibility by transformation with AlSRKb-SCRb, bringing to five the number of accessions in which self-fertility is due to, and was likely caused by, S-locus inactivation. Analysis of S-haplotype organization reveals that inter-haplotypic recombination events, rearrangements, and deletions have restructured the S locus and its genes in these accessions. We also perform a Quantitative Trait Loci (QTL) analysis to identify modifier loci associated with self-fertility in the Col-0 reference accession, which cannot be reverted to full self-incompatibility. Our results indicate that the transition to inbreeding occurred by at least two, and possibly more, independent S-locus mutations, and identify a novel unstable modifier locus that contributes to self-fertility in Col-0.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Goddard MR, Godfray HC, Burt A. Sex increases the efficacy of natural selection in experimental yeast populations. Nature. 2005;434:636–640. - PubMed
-
- Zeyl C, Bell G. The advantage of sex in evolving yeast populations. Nature. 1997;388:465–468. - PubMed
-
- Paland S, Lynch M. Transitions to asexuality result in excess amino acid substitutions. Science. 2006;311:990–992. - PubMed
-
- Barrett SC. The evolution of plant sexual diversity. Nat Rev Genet. 2002;3:274–284. - PubMed
-
- Stebbins GL. Self-fertilization and population variability in the higher plants. Am Nat. 1957;91:337–354.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

