Activation of host translational control pathways by a viral developmental switch
- PMID: 19300492
- PMCID: PMC2652079
- DOI: 10.1371/journal.ppat.1000334
Activation of host translational control pathways by a viral developmental switch
Abstract
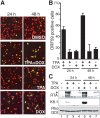
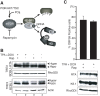
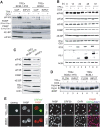
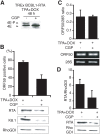
In response to numerous signals, latent herpesvirus genomes abruptly switch their developmental program, aborting stable host-cell colonization in favor of productive viral replication that ultimately destroys the cell. To achieve a rapid gene expression transition, newly minted capped, polyadenylated viral mRNAs must engage and reprogram the cellular translational apparatus. While transcriptional responses of viral genomes undergoing lytic reactivation have been amply documented, roles for cellular translational control pathways in enabling the latent-lytic switch have not been described. Using PEL-derived B-cells naturally infected with KSHV as a model, we define efficient reactivation conditions and demonstrate that reactivation substantially changes the protein synthesis profile. New polypeptide synthesis correlates with 4E-BP1 translational repressor inactivation, nuclear PABP accumulation, eIF4F assembly, and phosphorylation of the cap-binding protein eIF4E by Mnk1. Significantly, inhibiting Mnk1 reduces accumulation of the critical viral transactivator RTA through a post-transcriptional mechanism, limiting downstream lytic protein production, and impairs reactivation efficiency. Thus, herpesvirus reactivation from latency activates the host cap-dependent translation machinery, illustrating the importance of translational regulation in implementing new developmental instructions that drastically alter cell fate.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Ron D, Harding HP. eIF2α phosphorylation in cellular stress responses and disease. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational control in biology and medicine. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2007. pp. 345–368.
-
- Thompson B, Wickens M, Kimble J. Translational control in development. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational control in biology and medicine. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2007. pp. 507–544.
-
- Raught B, Gingras AC. Signaling to translation initiation. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational control in biology and medicine. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2007. pp. 369–400.
-
- Morley SJ, Rau M, Kay JE, Pain VM. Increased phosphorylation of eukaryotic initiation factor 4 {alpha} during activation of T lymphocytes correlates with increased eIF-4F complex formation. Eur J Biochem. 1993;218:39–48. - PubMed
-
- Beretta L, Singer NG, Hinderer R, Gingras AC, Richardson B, et al. Differential regulation of translation and eIF4E phosphorylation during human thymocyte maturation. J Immunol. 1998;160:3269–3273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

