A plant germline-specific integrator of sperm specification and cell cycle progression
- PMID: 19300502
- PMCID: PMC2653642
- DOI: 10.1371/journal.pgen.1000430
A plant germline-specific integrator of sperm specification and cell cycle progression
Abstract
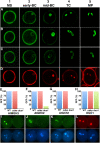
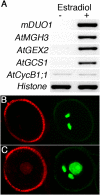
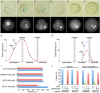
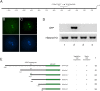
The unique double fertilisation mechanism in flowering plants depends upon a pair of functional sperm cells. During male gametogenesis, each haploid microspore undergoes an asymmetric division to produce a large, non-germline vegetative cell and a single germ cell that divides once to produce the sperm cell pair. Despite the importance of sperm cells in plant reproduction, relatively little is known about the molecular mechanisms controlling germ cell proliferation and specification. Here, we investigate the role of the Arabidopsis male germline-specific Myb protein DUO POLLEN1, DUO1, as a positive regulator of male germline development. We show that DUO1 is required for correct male germ cell differentiation including the expression of key genes required for fertilisation. DUO1 is also necessary for male germ cell division, and we show that DUO1 is required for the germline expression of the G2/M regulator AtCycB1;1 and that AtCycB1:1 can partially rescue defective germ cell division in duo1. We further show that the male germline-restricted expression of DUO1 depends upon positive promoter elements and not upon a proposed repressor binding site. Thus, DUO1 is a key regulator in the production of functional sperm cells in flowering plants that has a novel integrative role linking gametic cell specification and cell cycle progression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Honys D, Oh SA, Twell D. Malho R, editor. Pollen development, a genetic and transcriptomic view. The Pollen Tube: A Cellular and Molecular Perspective. 2006. pp. 15–45. Springer-Verlag- Berlin. Plant Cell Monographs. Volume.
-
- Strome S, Lehmann R. Germ versus soma decisions: lessons from flies and worms. Science. 2007;316:392–393. - PubMed
-
- Engel ML, Chaboud A, Dumas C, McCormick S. Sperm cells of Zea mays have a complex complement of mRNAs. Plant J. 2003;34:697–707. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

