Repetitive transcranial magnetic stimulation for treatment of elderly patients with depression - an open label trial
- PMID: 19300628
- PMCID: PMC2656335
Repetitive transcranial magnetic stimulation for treatment of elderly patients with depression - an open label trial
Abstract
Background: RTMS has been developed as a novel tool for treating depression but the clinical significance of this treatment has been variable, especially in the older depressed subjects.
Methods: Medication-resistant depressed patients 60 years or older were treated for two weeks (10 sessions) with high-frequency rTMS delivered to the left dorsolateral prefrontal cortex at 100% of motor threshold. Each session consisted of 20 trains at 10Hz delivered in 8-second duration. The patients continued taking their psychotropic medications throughout the study.
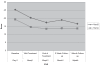
Results: Nineteen of the 20 subjects completed the trial. One subject dropped out after 8 sessions because of discomfort. The average age of our patients was 66.8 years (6 males and 14 females). Six patients responded and there was a 31.6% mean reduction in Hamilton Depression Rating Scale (HDRS) scores from baseline at the end of the treatment. There was statistically significant decrease from baseline in both HDRS and HARS scores at the end of treatment. rTMS was generally well tolerated.
Conclusion: These preliminary finding suggests that rTMS may be an effective treatment alternative to a subpopulation of medication resistant older depressed patients.
Keywords: depressed elderly; transcranial magnetic stimulation.
Figures
References
-
- American Psychiatric Association. Diagnostic and statistical manual for mental disorders (DSM-IV-TR) 4th Ed Text Revision edn. Washington, DC: 2000.
-
- Avery DH, Holtzheimer PE, 3rd, Fawaz W, et al. A controlled study of repetitive transcranial magnetic stimulation in medication-resistant major depression. Biol Psychiatry. 2006;59:187–94. - PubMed
-
- Baldwin RC, Simpson S. Treatment resistant depression in the elderly: a review of its conceptualization, management and relationship to organic brain disease. J Affect Disord. 1997;46:163–73. - PubMed
-
- Beck AT, Ward CH, Mendelsohn M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. - PubMed
-
- Beekman ATF, Penninx BWJH, Deeg DJH, et al. The impact of depression on the well-being, disability and use of services in older adults: a longitudinal perspective. Acta Psychiatr Scand. 2002;105:20–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources