SWISSspine: a nationwide registry for health technology assessment of lumbar disc prostheses
- PMID: 19301042
- PMCID: PMC2899652
- DOI: 10.1007/s00586-009-0934-8
SWISSspine: a nationwide registry for health technology assessment of lumbar disc prostheses
Erratum in
- Eur Spine J. 2009 Jun;18(6):862
Abstract
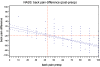
SWISSspine is a so-called pragmatic trial for assessment of safety and efficiency of total disc arthroplasty (TDA). It follows the new health technology assessment (HTA) principle of "coverage with evidence development". It is the first mandatory HTA registry of its kind in the history of Swiss orthopaedic surgery. Its goal is the generation of evidence for a decision by the Swiss federal office of health about reimbursement of the concerned technologies and treatments by the basic health insurance of Switzerland. During the time between March 2005 and 2008, 427 interventions with implantation of 497 lumbar total disc arthroplasties have been documented. Data was collected in a prospective, observational multicenter mode. The preliminary timeframe for the registry was 3 years and has already been extended. Data collection happens pre- and perioperatively, at the 3 months and 1-year follow-up and annually thereafter. Surgery, implant and follow-up case report forms are administered by spinal surgeons. Comorbidity questionnaires, NASS and EQ-5D forms are completed by the patients. Significant and clinically relevant reduction of low back pain VAS (70.3-29.4 points preop to 1-year postop, p < 0.0001) leg pain VAS (55.5-19.1 points preop to 1-year postop, p < 0.001), improvement of quality of life (EQ-5D, 0.32-0.73 points preop to 1-year postop, p < 0.001) and reduction of pain killer consumption was revealed at the 1-year follow-up. There were 14 (3.9%) complications and 7 (2.0%) revisions within the same hospitalization reported for monosegmental TDA; there were 6 (8.6%) complications and 8 (11.4%) revisions for bisegmental surgery. There were 35 patients (9.8%) with complications during followup in monosegmental and 9 (12.9%) in bisegmental surgery and 11 (3.1%) revisions with 1 [corrected] new hospitalization in monosegmental and 1 (1.4%) in bisegmental surgery. Regression analysis suggested a preoperative VAS "threshold value" of about 44 points for increased likelihood of a minimum clinically relevant back pain improvement. In a short-term perspective, lumbar TDA appears as a relatively safe and efficient procedure concerning pain reduction and improvement of quality of life. Nevertheless, no prediction about the long-term goals of TDA can be made yet. The SWISSspine registry proofs to be an excellent tool for collection of observational data in a nationwide framework whereby advantages and deficits of its design must be considered. It can act as a model for similar projects in other health-care domains.
Figures


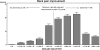



References
-
- Bertagnoli R, Yue JJ, Shah RV, Nanieva R, Pfeiffer F, Fenk-Mayer A, Kershaw T, Husted DS. The treatment of disabling single-level lumbar discogenic low back pain with total disc arthroplasty utilizing the prodisc prosthesis: a prospective study with 2-year minimum follow-up. Spine. 2005;30:2230–2236. doi: 10.1097/01.brs.0000182217.87660.40. - DOI - PubMed

