Kit and foxd3 genetically interact to regulate melanophore survival in zebrafish
- PMID: 19301400
- PMCID: PMC2730777
- DOI: 10.1002/dvdy.21910
Kit and foxd3 genetically interact to regulate melanophore survival in zebrafish
Abstract
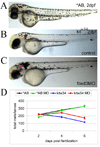
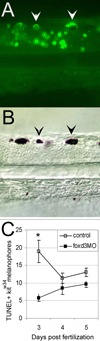
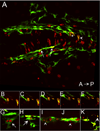
We have investigated the role of foxd3 activity in conjunction with signaling by the kit tyrosine kinase receptor in zebrafish black pigment cell (melanophore) development. As loss-of-function of these molecules individually has distinct effects on melanophore number, we have examined the phenotype of double mutants. Individuals with a null mutation in kit have fewer melanophores than wild-type, with cells lost through death. When kit mutants are injected with foxd3 antisense morpholino oligonucleotides or crossed with a foxd3 zebrafish mutant, they have more melanophores than their uninjected or foxd3+ counterparts. Examination of foxd3 loss-of-function in two additional kit mutants that differentially alter kit-dependent migration and survival indicates a change in melanophore number in survival mutants only. Consistently, TUNEL (terminal deoxynucleotidyl transferase-mediated deoxyuridinetriphosphate nick end-labeling) analysis confirms a partial rescue of melanophores from cell death. Ectopic expression of foxd3 indicates that foxd3 promotes early melanophore death only when kit is inactive. Taken together, these data suggest a kit-dependent role for foxd3 in the regulation of melanophore survival.
Copyright 2009 Wiley-Liss, Inc.
Figures





References
-
- Alkhateeb A, Fain PR, Spritz RA. Candidate functional promoter variant in the FOXD3 melanoblast developmental regulator gene in autosomal dominant vitiligo. Journal of Investigative Dermatology. 2005;125:388–391. - PubMed
-
- Bernex F, De Sepulveda P, Kress C, Elbaz C, Delouis C, Panthier JJ. Spatial and temporal patterns of c-kit-expressing cells in WlacZ/+ and WlacZ/WlacZ mouse embryos. Development. 1996;122:3023–3033. - PubMed
-
- Blechman JM, Lev S, Brizzi MF, Leitner O, Pegoraro L, Givol D, Yarden Y. Soluble c-kit proteins and antireceptor monoclonal antibodies confine the binding site of the stem cell factor. Journal of Biological Chemistry. 1993;268:4399–4406. - PubMed
-
- Copeland NG, Gilbert DJ, Cho BC, Donovan PJ, Jenkins NA, Cosman D, Anderson D, Lyman SD, Williams DE. Mast cell growth factor maps near the steel locus on mouse chromosome 10 and is deleted in a number of steel alleles. Cell. 1990;63:175–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

