Functional nucleic acid sensors
- PMID: 19301873
- PMCID: PMC2681788
- DOI: 10.1021/cr030183i
Functional nucleic acid sensors
Figures


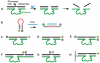





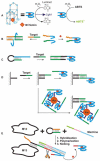



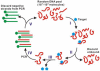

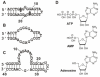









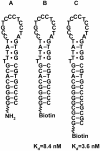
References
-
- Tsien RY. In: Fluorescenct Chemosensors for Ion and Molecule Recognization. 538 ed. Czarnik AW, editor. American Chemical Society; Washington, DC: 1993.
-
- Czarnik AW. Chem. Biol. 1995;2:423. - PubMed
-
- Iqbal SS, Mayo MW, Bruno JG, Bronk BV, Batt CA, Chambers JP. Biosens. Bioelectron. 2000;15:549. - PubMed
-
- Ligler FS, Rowe Taitt CA, editors. Optical Biosensors:Present and Future. Elsevier Science B.V; Amsterdam: 2002.
-
- Robertson DL, Joyce GF. Nature. 1990;344:467. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

