Cellular senescence: its role in tumor suppression and aging
- PMID: 19302284
- PMCID: PMC11158751
- DOI: 10.1111/j.1349-7006.2009.01123.x
Cellular senescence: its role in tumor suppression and aging
Abstract
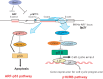
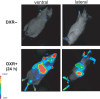
In normal tissue, cell division is carefully regulated to maintain the correct proliferative balance. Abnormal cell division underlies many hypoproliferative and hyperproliferative disorders, including cancer, and a better understanding of the mechanisms involved could lead to new strategies for treatment and prevention. Cellular senescence, a state of irreversible growth arrest, was first described as a limit to the replicative life span of somatic cells after serial cultivation in vitro. Recently, however, it has also been shown to be triggered prematurely by potentially oncogenic stimuli such as oncogene expression, oxidative stress, and DNA damage in cell culture studies. These data suggest that cellular senescence is therefore acting as a tumor-protective fail-safe mechanism. However, the significance of cellular senescence has remained an issue of debate over the years, with the possibility that it might be a cell culture-related artifact. Recent reports on oncogene-induced senescence detected in premalignant tumors have provided evidence to validate its role as a physiological response to prevent oncogenesis in vivo. In this review, we discuss the mechanisms for cellular senescence and its roles in vivo.
Figures


References
-
- Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res 1961; 25: 585–621. - PubMed
-
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 2007; 9: 729–40. - PubMed
-
- Wright WE, Shay JW. Telomere dynamics in cancer progression and prevention: fundamental differences in human and mouse telomere biology. Nat Med 2000; 6: 849–51. - PubMed
-
- Serrano M, Blasco MA. Putting the stress on senescence. Curr Opin Cell Biol 2001; 13: 748–53. - PubMed
-
- Collado M, Gil J, Efeyan A et al . Tumor biology: senescence in premalignant tumors. Nature 2005; 436: 642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

