Leukemia-related transcription factor TEL/ETV6 expands erythroid precursors and stimulates hemoglobin synthesis
- PMID: 19302286
- PMCID: PMC11158721
- DOI: 10.1111/j.1349-7006.2009.01097.x
Leukemia-related transcription factor TEL/ETV6 expands erythroid precursors and stimulates hemoglobin synthesis
Abstract
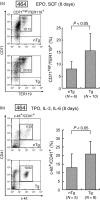
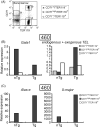
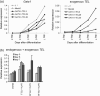
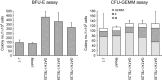
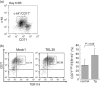
TEL/ETV6 located at chromosome 12p13 encodes a member of the E26 transformation-specific family of transcription factors. TEL is known to be rearranged in a variety of leukemias and solid tumors resulting in the formation of oncogenic chimeric protein. Tel is essential for maintaining hematopoietic stem cells in the bone marrow. To understand the role of TEL in erythropoiesis, we generated transgenic mice expressing human TEL under the control of Gata1 promoter that is activated during the course of the erythroid-lineage differentiation (GATA1-TEL transgenic mice). Although GATA1-TEL transgenic mice appeared healthy up to 18 months of age, the level of hemoglobin was higher in transgenic mice compared to non-transgenic littermates. In addition, CD71+/TER119+ and c-kit+/CD41+ populations proliferated with a higher frequency in transgenic mice when bone marrow cells were cultured in the presence of erythropoietin and thrombopoietin, respectively. In transgenic mice, enhanced expression of Alas-e and beta-major globin genes was observed in erythroid-committed cells. When embryonic stem cells expressing human TEL under the same Gata1 promoter were differentiated into hematopoietic cells, immature erythroid precursor increased better compared to controls as judged from the numbers of burst-forming unit of erythrocytes. Our findings suggest some roles of TEL in expanding erythroid precursors and accumulating hemoglobin.
Figures

Similar articles
-
ETV6 in hematopoiesis and leukemia predisposition.Semin Hematol. 2017 Apr;54(2):98-104. doi: 10.1053/j.seminhematol.2017.04.005. Epub 2017 Apr 7. Semin Hematol. 2017. PMID: 28637624 Free PMC article. Review.
-
TEL/ETV6 accelerates erythroid differentiation and inhibits megakaryocytic maturation in a human leukemia cell line UT-7/GM.Cancer Sci. 2005 Jun;96(6):340-8. doi: 10.1111/j.1349-7006.2005.00052.x. Cancer Sci. 2005. PMID: 15958056 Free PMC article.
-
Erythroid-specific activation of the distal (testis) promoter of GATA1 during differentiation of purified normal murine hematopoietic stem cells.Acta Haematol. 1996;95(3-4):229-35. doi: 10.1159/000203883. Acta Haematol. 1996. PMID: 8677748
-
The TEL/ETV6 gene is required specifically for hematopoiesis in the bone marrow.Genes Dev. 1998 Aug 1;12(15):2392-402. doi: 10.1101/gad.12.15.2392. Genes Dev. 1998. PMID: 9694803 Free PMC article.
-
[Role of TEL gene alterations in childhood leukemias].Rinsho Ketsueki. 2011 Aug;52(8):686-94. Rinsho Ketsueki. 2011. PMID: 21897076 Review. Japanese. No abstract available.
Cited by
-
Common genetic variation in ETV6 is associated with colorectal cancer susceptibility.Nat Commun. 2016 May 5;7:11478. doi: 10.1038/ncomms11478. Nat Commun. 2016. PMID: 27145994 Free PMC article.
-
Germline ETV6 mutations and predisposition to hematological malignancies.Int J Hematol. 2017 Aug;106(2):189-195. doi: 10.1007/s12185-017-2259-4. Epub 2017 May 29. Int J Hematol. 2017. PMID: 28555414 Review.
-
ETV6 in hematopoiesis and leukemia predisposition.Semin Hematol. 2017 Apr;54(2):98-104. doi: 10.1053/j.seminhematol.2017.04.005. Epub 2017 Apr 7. Semin Hematol. 2017. PMID: 28637624 Free PMC article. Review.
-
ETV6 (TEL1) regulates embryonic hematopoiesis in zebrafish.Haematologica. 2015 Jan;100(1):23-31. doi: 10.3324/haematol.2014.104091. Epub 2014 Oct 3. Haematologica. 2015. PMID: 25281506 Free PMC article.
-
Zebrafish ETV7 regulates red blood cell development through the cholesterol synthesis pathway.Dis Model Mech. 2014 Feb;7(2):265-70. doi: 10.1242/dmm.012526. Epub 2013 Dec 19. Dis Model Mech. 2014. PMID: 24357328 Free PMC article.
References
-
- Mavrothalassitis G, Ghysdael J. Proteins of the ETS family with transcriptional repressor activity. Oncogene 2000; 19: 6524–32. - PubMed
-
- Bohlander SK. ETV6: a versatile player in leukemogenesis. Semin Cancer Biol 2005; 15: 162–74. - PubMed
-
- Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets‐like gene, Tel., in chronic myelomonocytic leukemia with t (5;12) chromosomal translocation. Cell 1994; 77: 307–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

