Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity
- PMID: 19302289
- PMCID: PMC11158185
- DOI: 10.1111/j.1349-7006.2009.01115.x
Platelets and fibrinogen facilitate each other in protecting tumor cells from natural killer cytotoxicity
Abstract
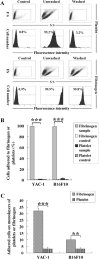
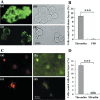
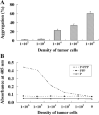
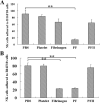
The functions of platelets and fibrinogen in protecting tumor cells from natural killer cytotoxicity have been discussed for more than 20 years. However, their exact roles and relationships in the process are still not clear. In this study, we show that tumor cells prefer to adhere to fibrinogen than to platelets, and fibrinogen can enhance the adhesion of tumor cells to platelets. Beta3 integrin plays an important role in the adhesion of B16F10 to platelets enhanced by fibrinogen. In the presence of thrombin, fibrinogen forms dense fibrin(ogen) layers around tumor cells. Tumor cells can induce platelets to aggregate and form thrombin. Platelets, as well as thrombin, can help fibrinogen protect tumor cells from lethal contact with natural killer cells and natural killer cytotoxicity. Hirudin, a specific inhibitor of thrombin, can reverse the effect of platelets on fibrinogen in blocking natural killer cytotoxicity. Our results suggest that fibrinogen helps platelets to adhere to tumor cells, and platelets in turn promote more fibrinogen to aggregate around tumor cells by forming thrombin. They facilitate each other in protecting tumor cells from natural killer cytotoxicity.
Figures


Similar articles
-
Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells.Blood. 2005 Jan 1;105(1):178-85. doi: 10.1182/blood-2004-06-2272. Epub 2004 Sep 14. Blood. 2005. PMID: 15367435
-
Unstimulated and thrombin-stimulated platelets binding to immobilized fibrinogen and fibrin on polystyrene supports.Haemostasis. 1995 Jul-Aug;25(4):158-65. doi: 10.1159/000217156. Haemostasis. 1995. PMID: 7557654
-
Deposition of fibrinogen on the surface of in vitro thrombi prevents platelet adhesion.Thromb Res. 2015 Dec;136(6):1231-9. doi: 10.1016/j.thromres.2015.10.001. Epub 2015 Oct 9. Thromb Res. 2015. PMID: 26482763 Free PMC article.
-
Thrombin-Fibrin(ogen) Interactions, Host Defense and Risk of Thrombosis.Int J Mol Sci. 2021 Mar 4;22(5):2590. doi: 10.3390/ijms22052590. Int J Mol Sci. 2021. PMID: 33806700 Free PMC article. Review.
-
Modulation of natural killer cell anti-tumor reactivity by platelets.J Innate Immun. 2011;3(4):374-82. doi: 10.1159/000323936. Epub 2011 Mar 12. J Innate Immun. 2011. PMID: 21411974 Review.
Cited by
-
Holothurian glycosaminoglycan inhibits metastasis and thrombosis via targeting of nuclear factor-κB/tissue factor/Factor Xa pathway in melanoma B16F10 cells.PLoS One. 2013;8(2):e56557. doi: 10.1371/journal.pone.0056557. Epub 2013 Feb 21. PLoS One. 2013. PMID: 23437168 Free PMC article.
-
Prognostic Value of Plasma Fibrinogen in Lung Cancer Patients: A Meta-Analysis.J Cancer. 2018 Oct 10;9(21):3904-3911. doi: 10.7150/jca.26360. eCollection 2018. J Cancer. 2018. PMID: 30410594 Free PMC article.
-
Prognostic Value of Fibrinogen to Prealbumin Ratio (FPR) in Resectable Gastric Cancer.J Inflamm Res. 2024 Feb 27;17:1325-1335. doi: 10.2147/JIR.S440832. eCollection 2024. J Inflamm Res. 2024. PMID: 38434582 Free PMC article.
-
Prognostic and clinicopathological role of pretreatment fibrinogen-to-albumin ratio (FAR) in patients with gastric cancer: a meta-analysis.World J Surg Oncol. 2025 Jun 10;23(1):227. doi: 10.1186/s12957-025-03885-0. World J Surg Oncol. 2025. PMID: 40495195 Free PMC article.
-
A Nomogram Model Involving Preoperative Fibrinogen and Prognostic Nutritional Index Score for Predicting Postoperative Outcome in Patients with Gastric Cancer.Cancer Manag Res. 2021 May 25;13:4191-4201. doi: 10.2147/CMAR.S311347. eCollection 2021. Cancer Manag Res. 2021. PMID: 34079372 Free PMC article.
References
-
- Liotta LA. Cancer cell invasion and metastasis. Sci Am 1992; 266: 54–9, 62–3. - PubMed
-
- Fidler I. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125I‐5 iodo‐2‐deoxyuridine. J Natl Cancer Inst 1970; 45: 773–9. - PubMed
-
- Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium‐attached tumor cells: a new model for metastasis. Nat Med 2000; 6: 100–2. - PubMed
-
- Gorelik E, Wiltrout RH, Okumura K, Habu S, Herberman RB. Role of NK cells in the control of metastatic spread and growth of tumor cells in mice. Int J Cancer 1982; 30: 107–12. - PubMed
-
- Hanna N. The role of natural killer cells in the control of tumor growth and metastasis. Biochim Biophys Acta 1985; 780: 213–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

