Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma
- PMID: 19302292
- PMCID: PMC11159838
- DOI: 10.1111/j.1349-7006.2009.01106.x
Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma
Abstract
The identification of novel tumor-specific proteins or antigens is of great importance for diagnostic and therapeutic applications in pancreatic cancer. Using oligonucleotide microarrays, we identified a broad spectrum of differentially expressed pancreatic cancer-related genes. Of these, we selected an overexpressed expressed sequence taq and cloned a 721-bp full-length cDNA with an open reading frame of 196 amino acids. This novel gene was localized on the Homo sapiens 16p13.3 chromosomal locus, and its nucleotide sequence matched the Homo sapiens similar to common salivary protein 1 (LOC124220). We named the gene pancreatic adenocarcinoma up-regulated factor. The pancreatic adenocarcinoma up-regulated factor was secreted into the culture medium of pancreatic adenocarcinoma up-regulated factor-overexpressing Chinese hamster ovary cells, had an apparent molecular mass of approximately 25 kDa, and was N-glycosylated. The induction of pancreatic adenocarcinoma up-regulated factor in Chinese hamster ovary cells increased cell proliferation, migration, and invasion ability in vitro. Subcutaneous injection of mice with Chinese hamster ovary/pancreatic adenocarcinoma up-regulated factor cells resulted in 3.8-fold greater tumor sizes compared to Chinese hamster ovary/mock cells. Reverse transcription-polymerase chain reaction and western blotting with antirecombinant human pancreatic adenocarcinoma up-regulated factor antibodies confirmed that pancreatic adenocarcinoma up-regulated factor was highly expressed in six of eight pancreatic cancer cell lines. Immunohistochemical staining of human pancreatic cancer tissues also showed pancreatic adenocarcinoma up-regulated factor overexpression in the cytoplasm of cancer cells. Transfection with pancreatic adenocarcinoma up-regulated factor-specific small-interfering RNA reduced cancer cell migration and invasion in vitro. Treatment with antirecombinant human pancreatic adenocarcinoma up-regulated factor in vitro and in vivo reduced proliferation, migration, invasion, and tumorigenic ability. Collectively, our results suggest that pancreatic adenocarcinoma up-regulated factor is a novel secretory protein involved in pancreatic cancer progression and might be a potential target for the treatment of pancreatic cancer.
Figures

 ), and the canonical 3′‐polyadenylation signal AATAAA is located at nucleotide positions 698–703 (_). PAUF encodes a 196‐amino acid protein, including a 40‐amino acid signal peptide (bold type; cleavage site, bold arrow) with putative post‐translational modification sites. Specifically, one N‐glycosylation site at amino acids 185–189 (
), and the canonical 3′‐polyadenylation signal AATAAA is located at nucleotide positions 698–703 (_). PAUF encodes a 196‐amino acid protein, including a 40‐amino acid signal peptide (bold type; cleavage site, bold arrow) with putative post‐translational modification sites. Specifically, one N‐glycosylation site at amino acids 185–189 ( ); three phosphorylation sites at amino acids 7–9, 53–56, and 125–128 (
); three phosphorylation sites at amino acids 7–9, 53–56, and 125–128 ( ); and three N‐myristorylation sites at amino acids 64–69, 90–95, and 137–143 (
); and three N‐myristorylation sites at amino acids 64–69, 90–95, and 137–143 ( ) were predicted in silico. (b) The amino acid sequences of human PAUF, chimpanzee XP_523271 (similar to HRPE773), rhesus monkey XP_001086870 (similar to zymogen granule membrane protein 16 isoform 2), and mouse NP_064294 (demilone cell and parotid protein [Dcpp1]) were aligned by CLUSTAL W (1.83) multiple sequence alignment. Amino acid identities in all sequences are indicated by an asterisk while conserved and semiconserved substitutions are shown by a colon and a dot, respectively.
) were predicted in silico. (b) The amino acid sequences of human PAUF, chimpanzee XP_523271 (similar to HRPE773), rhesus monkey XP_001086870 (similar to zymogen granule membrane protein 16 isoform 2), and mouse NP_064294 (demilone cell and parotid protein [Dcpp1]) were aligned by CLUSTAL W (1.83) multiple sequence alignment. Amino acid identities in all sequences are indicated by an asterisk while conserved and semiconserved substitutions are shown by a colon and a dot, respectively.
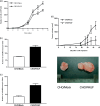
 ) cells showed a higher proliferation rate compared to CHO/Mock cells (
) cells showed a higher proliferation rate compared to CHO/Mock cells ( ). (b) Cell migration ability was assessed based on the number of cells per microscopic field (×100) that migrated through the pores of a Transwell chamber to the under surface of the membrane after an incubation time of 24 h. In CHO/PAUF cells, cell migration increased compared to CHO/Mock cells. (c) Cell invasion ability was assessed by counting the number of cells per microscopic field (×100) that migrated through Matrigel invasion chambers after 48 h. Cell invasion ability increased in CHO/PAUF cells compared to CHO/Mock cells. (d) The CHO/PAUF cells were injected into the flank of 6‐week‐old female Balb/c nude mice. The CHO/Mock cells were used as a control. Mice were monitored for 32 days after injection (n = 5 for each). Data are shown as the mean ± SEM. Representative images of tumor xenografts after 32 days shows that grafting with CHO/PAUF cells produced larger tumors than CHO/Mock treatments.
). (b) Cell migration ability was assessed based on the number of cells per microscopic field (×100) that migrated through the pores of a Transwell chamber to the under surface of the membrane after an incubation time of 24 h. In CHO/PAUF cells, cell migration increased compared to CHO/Mock cells. (c) Cell invasion ability was assessed by counting the number of cells per microscopic field (×100) that migrated through Matrigel invasion chambers after 48 h. Cell invasion ability increased in CHO/PAUF cells compared to CHO/Mock cells. (d) The CHO/PAUF cells were injected into the flank of 6‐week‐old female Balb/c nude mice. The CHO/Mock cells were used as a control. Mice were monitored for 32 days after injection (n = 5 for each). Data are shown as the mean ± SEM. Representative images of tumor xenografts after 32 days shows that grafting with CHO/PAUF cells produced larger tumors than CHO/Mock treatments.
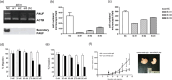
 ) or normal rabbit IgG (
) or normal rabbit IgG ( ). AntirhPAUF antibodies effectively inhibited the migration of CFPAC‐1 in a dose‐dependent manner. (e) CFPAC‐1 cells were plated on Matrigel‐coated filters and incubated for 24 h in the presence of the indicated concentration of antirhPAUF antibodies (
). AntirhPAUF antibodies effectively inhibited the migration of CFPAC‐1 in a dose‐dependent manner. (e) CFPAC‐1 cells were plated on Matrigel‐coated filters and incubated for 24 h in the presence of the indicated concentration of antirhPAUF antibodies ( ) or normal rabbit IgG (
) or normal rabbit IgG ( ). Invasion through Matrigel‐coated membranes was dose‐dependently inhibited by antirhPAUF antibodies. (f) Tumor‐bearing mice generated by the injection of CFPAC‐1 were treated with antirhPAUF antibodies (
). Invasion through Matrigel‐coated membranes was dose‐dependently inhibited by antirhPAUF antibodies. (f) Tumor‐bearing mice generated by the injection of CFPAC‐1 were treated with antirhPAUF antibodies ( ) or normal rabbit IgG (
) or normal rabbit IgG ( ) via the tail vein twice a week (n = 6 for each) and tumor mass was monitored (left) for 32 days. Data are shown as the mean ± SEM. The starting day of antibody injection is represented by the arrow. The representative image shows the results of tumor xenografts at the end of the experiments.
) via the tail vein twice a week (n = 6 for each) and tumor mass was monitored (left) for 32 days. Data are shown as the mean ± SEM. The starting day of antibody injection is represented by the arrow. The representative image shows the results of tumor xenografts at the end of the experiments.References
-
- Jemal A, Murray T, Ward E et al . Cancer Statistics, 2005. CA: A Cancer J Clin 2005; 55: 10–30. - PubMed
-
- Hawes RH, Xiong Q, Waxman I, Chang KJ, Evans DB, Abbruzzese JL. A multispecialty approach to the diagnosis and management of pancreatic cancer. Am J Gastroenterol 2000; 95: 17–31. - PubMed
-
- Hu YX, Watanabe H, Ohtsubo K et al . Frequent loss of p16 expression and its correlation with clinicopathological parameters in pancreatic carcinoma. Clin Cancer Res 1997; 3: 1473–7. - PubMed
-
- Dong M, Nio Y, Tamura K et al . Ki‐ras point mutation and p53 expression in human pancreatic cancer: a comparative study among Chinese, Japanese, and Western patients. Cancer Epidemiol Biomarkers Prev 2000; 9: 279–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

