Mutational analysis of the hepatitis C virus E1 glycoprotein in retroviral pseudoparticles and cell-culture-derived H77/JFH1 chimeric infectious virus particles
- PMID: 19302336
- PMCID: PMC2770242
- DOI: 10.1111/j.1365-2893.2009.01111.x
Mutational analysis of the hepatitis C virus E1 glycoprotein in retroviral pseudoparticles and cell-culture-derived H77/JFH1 chimeric infectious virus particles
Abstract
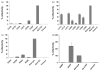
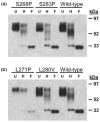
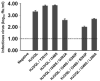
Cell entry by enveloped viruses is mediated by viral glycoproteins, and generally involves a short hydrophobic peptide (fusion peptide) that inserts into the cellular membrane. An internal hydrophobic domain within E1 (aa262-290) of hepatitis C virus (HCV) may function as a fusion peptide. Retrovirus-based HCV-pseudotyped viruses (HCVpp; genotype 1a) containing Ala or Pro substitutions at conserved amino acid positions within this putative fusion peptide were generated. Mutation of conserved residues significantly reduced efficiency of HCVpp entry into Huh-7 cells. The majority of amino acid substitutions appeared to disrupt necessary interactions between E1 and E2. For some mutants, reductions in HCVpp-associated E1 were associated with the incorporation of a high molecular weight, hyperglycosylated E2 that displayed decreased CD81-binding. Other entry-deficient mutants displayed normal E1E2 incorporation into pseudoparticles and normal CD81-binding, and therefore might affect viral fusion. One mutant (S283P) consistently displayed two- to threefold higher infectivity than did wild-type. Three mutations that decreased HCVpp infectivity also reduced levels of HCVcc infectious virus production. However, the S283P mutation had a different effect in the two systems as it did not increase production of infectious HCVcc. This comprehensive mutational analysis of the putative HCV fusion peptide provides insight into the role of E1 in its interaction with E2 and in HCV entry.
Figures


Similar articles
-
Dissecting the role of putative CD81 binding regions of E2 in mediating HCV entry: putative CD81 binding region 1 is not involved in CD81 binding.Virol J. 2008 Mar 20;5:46. doi: 10.1186/1743-422X-5-46. Virol J. 2008. PMID: 18355410 Free PMC article.
-
Analysis of a conserved RGE/RGD motif in HCV E2 in mediating entry.Virol J. 2009 Jan 26;6:12. doi: 10.1186/1743-422X-6-12. Virol J. 2009. PMID: 19171049 Free PMC article.
-
Functional Study of the C-Terminal Part of the Hepatitis C Virus E1 Ectodomain.J Virol. 2018 Sep 26;92(20):e00939-18. doi: 10.1128/JVI.00939-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30068644 Free PMC article.
-
Functional hepatitis C virus envelope glycoproteins.Biol Cell. 2004 Aug;96(6):413-20. doi: 10.1016/j.biolcel.2004.03.008. Biol Cell. 2004. PMID: 15325070 Review.
-
Hepatitis C virus entry: potential receptors and their biological functions.J Gen Virol. 2006 May;87(Pt 5):1075-1084. doi: 10.1099/vir.0.81646-0. J Gen Virol. 2006. PMID: 16603507 Review.
Cited by
-
Glycan Shielding and Modulation of Hepatitis C Virus Neutralizing Antibodies.Front Immunol. 2018 Apr 27;9:910. doi: 10.3389/fimmu.2018.00910. eCollection 2018. Front Immunol. 2018. PMID: 29755477 Free PMC article. Review.
-
A protein coevolution method uncovers critical features of the Hepatitis C Virus fusion mechanism.PLoS Pathog. 2018 Mar 5;14(3):e1006908. doi: 10.1371/journal.ppat.1006908. eCollection 2018 Mar. PLoS Pathog. 2018. PMID: 29505618 Free PMC article.
-
A Biologically-validated HCV E1E2 Heterodimer Structural Model.Sci Rep. 2017 Mar 16;7(1):214. doi: 10.1038/s41598-017-00320-7. Sci Rep. 2017. PMID: 28303031 Free PMC article.
-
Identification of interactions in the E1E2 heterodimer of hepatitis C virus important for cell entry.J Biol Chem. 2011 Jul 8;286(27):23865-76. doi: 10.1074/jbc.M110.213942. Epub 2011 May 9. J Biol Chem. 2011. PMID: 21555519 Free PMC article.
-
Novel functional hepatitis C virus glycoprotein isolates identified using an optimized viral pseudotype entry assay.J Gen Virol. 2016 Sep;97(9):2265-2279. doi: 10.1099/jgv.0.000537. Epub 2016 Jul 6. J Gen Virol. 2016. PMID: 27384448 Free PMC article.
References
-
- Lindenbach BD, Rice CM. In: Flaviviridae: the viruses and their replication. Knipe DM, Howley PM, editors. Fields Virology, Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 991–1042.
-
- Heinz FX, Allison SL. The machinery for flavivirus fusion with host cell membranes. Curr Opin Microbiol. 2001;4(4):450–455. - PubMed
-
- Kielian M. Class II virus membrane fusion proteins. Virology. 2006;344(1):38–47. - PubMed
-
- Op De Beeck A, Montserret R, Duvet S, et al. The transmembrane domains of hepatitis C virus envelope glycoproteins E1 and E2 play a major role in heterodimerization. J Biol Chem. 2000;275(40):31428–31437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

