Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma
- PMID: 19302559
- PMCID: PMC2704925
- DOI: 10.1111/j.1600-0609.2009.01257.x
Lenalidomide in combination with dexamethasone at first relapse in comparison with its use as later salvage therapy in relapsed or refractory multiple myeloma
Abstract
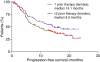
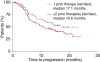
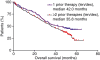
This subset analysis of data from two phase III studies in patients with relapsed or refractory multiple myeloma (MM) evaluated the benefit of initiating lenalidomide plus dexamethasone at first relapse. Multivariate analysis showed that fewer prior therapies, along with beta(2)-microglobulin (< or = 2.5 mg/L), predicted a better time to progression (TTP; study end-point) with lenalidomide plus dexamethasone treatment. Patients with one prior therapy showed a significant improvement in benefit after first relapse compared with those who received two or more therapies. Patients with one prior therapy had significantly prolonged median TTP (17.1 vs. 10.6 months; P = 0.026) and progression-free survival (14.1 vs. 9.5 months, P = 0.047) compared with patients treated in later lines. Overall response rates were higher (66.9% vs. 56.8%, P = 0.06), and the complete response plus very good partial response rate was significantly higher in first relapse (39.8% vs. 27.7%, P = 0.025). Importantly, overall survival was significantly prolonged for patients treated with lenalidomide plus dexamethasone with one prior therapy, compared with patients treated later in salvage (median of 42.0 vs. 35.8 months, P = 0.041), with no differences in toxicity, dose reductions, or discontinuations despite longer treatment. Therefore, lenalidomide plus dexamethasone is both effective and tolerable for second-line MM therapy and the data suggest that the greatest benefit occurs with earlier use.
Figures
References
-
- Terpos E, Rahemtulla A, Dimopoulos MA. Current treatment options for myeloma. Expert Opin Pharmacother. 2005;6:1127–42. - PubMed
-
- Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–42. - PubMed
-
- Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32. - PubMed
-
- Lacy MQ, Gertz MA, Dispenzieri A, et al. Long-term results of response to therapy, time to progression, and survival with lenalidomide plus dexamethasone in newly diagnosed myeloma. Mayo Clin Proc. 2007;82:1179–84. - PubMed

