Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses--relevance to cardiovascular pathologies and drug discovery
- PMID: 19302590
- PMCID: PMC2707963
- DOI: 10.1111/j.1476-5381.2009.00132.x
Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses--relevance to cardiovascular pathologies and drug discovery
Abstract
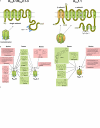
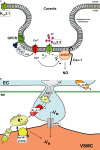
The arterial endothelium critically contributes to blood pressure control by releasing vasodilating autacoids such as nitric oxide, prostacyclin and a third factor or pathway termed 'endothelium-derived hyperpolarizing factor' (EDHF). The nature of EDHF and EDHF-signalling pathways is not fully understood yet. However, endothelial hyperpolarization mediated by the Ca(2+)-activated K(+) channels (K(Ca)) has been suggested to play a critical role in initializing EDHF-dilator responses in conduit and resistance-sized arteries of many species including humans. Endothelial K(Ca) currents are mediated by the two K(Ca) subtypes, intermediate-conductance K(Ca) (KCa3.1) (also known as, a.k.a. IK(Ca)) and small-conductance K(Ca) type 3 (KCa2.3) (a.k.a. SK(Ca)). In this review, we summarize current knowledge about endothelial KCa3.1 and KCa2.3 channels, their molecular and pharmacological properties and their specific roles in endothelial function and, particularly, in the EDHF-dilator response. In addition we focus on recent experimental evidences derived from KCa3.1- and/or KCa2.3-deficient mice that exhibit severe defects in EDHF signalling and elevated blood pressures, thus highlighting the importance of the KCa3.1/KCa2.3-EDHF-dilator system for blood pressure control. Moreover, we outline differential and overlapping roles of KCa3.1 and KCa2.3 for EDHF signalling as well as for nitric oxide synthesis and discuss recent evidence for a heterogeneous (sub) cellular distribution of KCa3.1 (at endothelial projections towards the smooth muscle) and KCa2.3 (at inter-endothelial borders and caveolae), which may explain their distinct roles for endothelial function. Finally, we summarize the interrelations of altered KCa3.1/KCa2.3 and EDHF system impairments with cardiovascular disease states such as hypertension, diabetes, dyslipidemia and atherosclerosis and discuss the therapeutic potential of KCa3.1/KCa2.3 openers as novel types of blood pressure-lowering drugs.
Figures

References
-
- Angulo J, Cuevas P, Fernandez A, Gabancho S, Allona A, Martin-Morales A, et al. Diabetes impairs endothelium-dependent relaxation of human penile vascular tissues mediated by NO and EDHF. Biochem Biophys Res Commun. 2003;312:1202–1208. - PubMed
-
- Atkinson NS, Robertson GA, Ganetzky B. A component of calcium-activated potassium channels encoded by the Drosophila slo locus. Science. 1991;253:551–555. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

