Myoendothelial coupling in the mesenteric arterial bed; segmental differences and interplay between nitric oxide and endothelin-1
- PMID: 19302591
- PMCID: PMC2697733
- DOI: 10.1111/j.1476-5381.2009.00128.x
Myoendothelial coupling in the mesenteric arterial bed; segmental differences and interplay between nitric oxide and endothelin-1
Abstract
Background and purpose: We tested the hypothesis that activated arterial smooth muscle (ASM) stimulates endothelial vasomotor influences via gap junctions and that the significance of this myoendothelial coupling increases with decreasing arterial diameter.
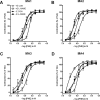
Experimental approach: From WKY rats, first-, second-, third- and fourth-order branches of the superior mesenteric artery (MA1, MA2, MA3 and MA4 respectively) were isolated and mounted in wire-myographs to record vasomotor responses to 0.16-20 micromol x L(-1) phenylephrine.
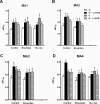
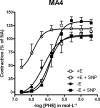
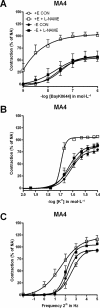
Key results: Removal of endothelium increased the sensitivity (pEC(50)) to phenylephrine in all arteries. The nitric oxide (NO) synthase inhibitor N(omega)-nitro-L-arginine methyl ester (L-NAME) (100 micromol x L(-1)) did not modify pEC(50) to phenylephrine in all denuded arteries, and increased it in intact MA1, MA2 and MA3 to the same extent as denudation. However, in intact MA4, the effect of L-NAME was significantly larger (DeltapEC(50) 0.57 +/- 0.02) than the effect of endothelium removal (DeltapEC(50) 0.20 +/- 0.06). This endothelium-dependent effect of L-NAME in MA4 was inhibited by (i) steroidal and peptidergic uncouplers of gap junctions; (ii) a low concentration of the NO donor sodium nitroprusside; and (iii) by the endothelin-receptor antagonist bosentan. It was also observed during contractions induced by (i) calcium channel activation (BayK 8644, 0.001-1 micromol x L(-1)); (ii) depolarization (10-40 mmol x L(-1) K(+)); and (iii) sympathetic nerve stimulation (0.25-32 Hz).
Conclusions and implications: These pharmacological observations indicated feedback control by endothelium of ASM reactivity involving gap junctions and a balance between endothelium-derived NO and endothelin-1. This myoendothelial coupling was most prominent in distal resistance arteries.
Figures
Similar articles
-
Blood pressure and vascular effects of endothelin blockade in chronic nitric oxide-deficient hypertension.Hypertension. 1997 Mar;29(3):763-9. doi: 10.1161/01.hyp.29.3.763. Hypertension. 1997. PMID: 9052893
-
An endothelium-derived hyperpolarizing factor-like factor moderates myogenic constriction of mesenteric resistance arteries in the absence of endothelial nitric oxide synthase-derived nitric oxide.Hypertension. 2001 Oct;38(4):833-9. doi: 10.1161/hy1001.092651. Hypertension. 2001. PMID: 11641295
-
An indirect influence of phenylephrine on the release of endothelium-derived vasodilators in rat small mesenteric artery.Br J Pharmacol. 2000 Jan;129(2):381-7. doi: 10.1038/sj.bjp.0703052. Br J Pharmacol. 2000. PMID: 10694246 Free PMC article.
-
Coordination of vasomotor responses by the endothelium.Circ J. 2010 Feb;74(2):226-32. doi: 10.1253/circj.cj-09-0879. Epub 2010 Jan 9. Circ J. 2010. PMID: 20065608 Review.
-
Inducible nitric oxide synthase and atherosclerosis.Clin Cardiol. 1998 Jul;21(7):473-6. doi: 10.1002/clc.4960210705. Clin Cardiol. 1998. PMID: 9669055 Free PMC article. Review.
Cited by
-
Deletion of endothelial arginase 1 does not improve vasomotor function in diabetic mice.Physiol Rep. 2018 Jun;6(11):e13717. doi: 10.14814/phy2.13717. Physiol Rep. 2018. PMID: 29890043 Free PMC article.
-
Matricellular protein thrombospondin-1 in pulmonary hypertension: multiple pathways to disease.Cardiovasc Res. 2017 Jul 1;113(8):858-868. doi: 10.1093/cvr/cvx094. Cardiovasc Res. 2017. PMID: 28472457 Free PMC article. Review.
-
Evolved changes in reflex control of the cardiovascular system in deer mice native to high altitude.J Exp Biol. 2025 Jun 15;228(12):jeb249483. doi: 10.1242/jeb.249483. Epub 2025 Jun 18. J Exp Biol. 2025. PMID: 40401751 Free PMC article.
-
Acidosis potentiates endothelium-dependent vasorelaxation and gap junction communication in the superior mesenteric artery.Eur J Pharmacol. 2018 May 15;827:22-31. doi: 10.1016/j.ejphar.2018.03.004. Epub 2018 Mar 7. Eur J Pharmacol. 2018. PMID: 29524386 Free PMC article.
-
20-Hydroxyeicosatetraenoic Acid (20-HETE) Modulates Canonical Transient Receptor Potential-6 (TRPC6) Channels in Podocytes.Front Physiol. 2016 Aug 31;7:351. doi: 10.3389/fphys.2016.00351. eCollection 2016. Front Physiol. 2016. PMID: 27630573 Free PMC article.
References
-
- Burnstock G, Ralevic V. New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br J Plast Surg. 1994;47:527–543. - PubMed
-
- Busse R, Fleming I. Regulation of endothelium-derived vasoactive autacoid production by hemodynamic forces. Trends Pharmacol Sci. 2003;24:24–29. - PubMed
-
- Busse R, Pohl U, Luckhoff A. Mechanisms controlling the release of endothelial autocoids. Z Kardiol. 1989;78:64–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

