Colony stimulating factor-1 dependence of paneth cell development in the mouse small intestine
- PMID: 19303020
- PMCID: PMC2706482
- DOI: 10.1053/j.gastro.2009.03.004
Colony stimulating factor-1 dependence of paneth cell development in the mouse small intestine
Abstract
Background & aims: Paneth cells (PCs) secrete defensins and antimicrobial enzymes that contribute to innate immunity against pathogen infections within the mucosa of the small intestine. We examined the role of colony stimulating factor-1 (CSF-1) in PC development.
Methods: CSF-1-deficient and CSF-1 receptor (CSF-1R)-deficient mice and administration of neutralizing anti-CSF-1R antibody were used to study the requirement of CSF-1 for the development of epithelial cells of the small intestine. CSF-1 transgenic reporter mice and mice that express only the membrane-spanning, cell-surface CSF-1 isoform were used to investigate regulation by systemic versus local CSF-1.
Results: Mice deficient in CSF-1 or CSF-1R had greatly reduced numbers of mature PCs. PCs express the CSF-1R, and administration of anti-CSF-1R antibody to neonatal mice significantly reduced the number of PCs. Analysis of transgenic CSF-1 reporter mice showed that CSF-1-expressing cells are in close proximity to PCs. CSF-1/CSF-1R-deficient mice also had reduced numbers of the proliferating epithelial cell progenitors and lamina propria macrophages. Expression of the membrane-spanning, cell-surface CSF-1 isoform in CSF-1-deficient mice completely rescued the deficiencies of PCs, proliferating progenitors, and lamina propria macrophages.
Conclusions: These results indicate local regulation by CSF-1 of PC development, either directly, in a juxtacrine/paracrine manner, or indirectly, by lamina propria macrophages. Therefore, CSF-1R hyperstimulation could be involved in hyperproliferative disorders of the small intestine, such as Crohn's disease and ulcerative colitis.
Conflict of interest statement
Figures
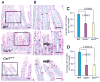


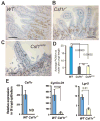



Comment in
-
Paneth cell development, differentiation, and function: new molecular cues.Gastroenterology. 2009 Jul;137(1):30-3. doi: 10.1053/j.gastro.2009.05.013. Epub 2009 Jun 2. Gastroenterology. 2009. PMID: 19497398 No abstract available.
Similar articles
-
Paneth cell development, differentiation, and function: new molecular cues.Gastroenterology. 2009 Jul;137(1):30-3. doi: 10.1053/j.gastro.2009.05.013. Epub 2009 Jun 2. Gastroenterology. 2009. PMID: 19497398 No abstract available.
-
The CSF-1 receptor fashions the intestinal stem cell niche.Stem Cell Res. 2013 Mar;10(2):203-12. doi: 10.1016/j.scr.2012.12.001. Epub 2012 Dec 9. Stem Cell Res. 2013. PMID: 23314290 Free PMC article.
-
Functional overlap but differential expression of CSF-1 and IL-34 in their CSF-1 receptor-mediated regulation of myeloid cells.J Leukoc Biol. 2010 Sep;88(3):495-505. doi: 10.1189/jlb.1209822. Epub 2010 May 26. J Leukoc Biol. 2010. PMID: 20504948 Free PMC article.
-
Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling.Blood. 2012 Feb 23;119(8):1810-20. doi: 10.1182/blood-2011-09-379214. Epub 2011 Dec 20. Blood. 2012. PMID: 22186992 Review.
-
Biology and action of colony--stimulating factor-1.Mol Reprod Dev. 1997 Jan;46(1):4-10. doi: 10.1002/(SICI)1098-2795(199701)46:1<4::AID-MRD2>3.0.CO;2-V. Mol Reprod Dev. 1997. PMID: 8981357 Review.
Cited by
-
Recent advances in small bowel diseases: Part I.World J Gastroenterol. 2012 Jul 14;18(26):3336-52. doi: 10.3748/wjg.v18.i26.3336. World J Gastroenterol. 2012. PMID: 22807604 Free PMC article. Review.
-
Pleiotropic Impacts of Macrophage and Microglial Deficiency on Development in Rats with Targeted Mutation of the Csf1r Locus.J Immunol. 2018 Nov 1;201(9):2683-2699. doi: 10.4049/jimmunol.1701783. Epub 2018 Sep 24. J Immunol. 2018. PMID: 30249809 Free PMC article.
-
Trans-eQTLs reveal that independent genetic variants associated with a complex phenotype converge on intermediate genes, with a major role for the HLA.PLoS Genet. 2011 Aug;7(8):e1002197. doi: 10.1371/journal.pgen.1002197. Epub 2011 Aug 4. PLoS Genet. 2011. PMID: 21829388 Free PMC article.
-
CSF-1 receptor signaling in myeloid cells.Cold Spring Harb Perspect Biol. 2014 Jun 2;6(6):a021857. doi: 10.1101/cshperspect.a021857. Cold Spring Harb Perspect Biol. 2014. PMID: 24890514 Free PMC article. Review.
-
Niche-specific functional heterogeneity of intestinal resident macrophages.Gut. 2021 Jul;70(7):1383-1395. doi: 10.1136/gutjnl-2020-323121. Epub 2020 Dec 31. Gut. 2021. PMID: 33384336 Free PMC article. Review.
References
-
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–61. - PubMed
-
- Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–36. - PubMed
-
- Sancho E, Batlle E, Clevers H. Live and let die in the intestinal epithelium. Curr Opin Cell Biol. 2003;15:763–70. - PubMed
-
- Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

