Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles
- PMID: 19303334
- PMCID: PMC2716660
- DOI: 10.1016/j.immuni.2009.01.011
Initial clonal expansion of germinal center B cells takes place at the perimeter of follicles
Abstract
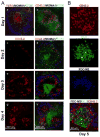
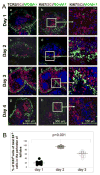
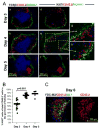
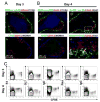
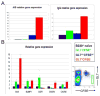
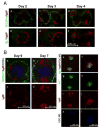
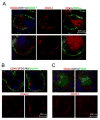
Current models of the germinal center (GC) response propose that after stimulation at the edges of T cell zones, pre-GC B cells directly migrate to the center of follicles and proliferate to form GCs. We followed the interrelationship of proliferation, differentiation, and microenvironmental locale in populations of pre-GC B cells responding to antigen. In contrast to the predictions of current models, after accumulation at the T-B interface, these cells appeared at the perimeter of follicles adjacent to the marginal zone. There, they rapidly proliferated for several days but underwent no V gene hypermutation and little heavy-chain class switching. Their chemokine receptor expression pattern indicated that these cells were sessile, yet they had begun to acquire many phenotypic characteristics of GC B cells. The expanded clones were subsequently observed in the center of follicles, suggesting that GCs are created by coalescence of B cells from this follicular perimeter response.
Figures
References
-
- Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;9:943–952. - PubMed
-
- Allen CD, Okada T, Tang HL, Cyster JG. Imaging of germinal center selection events during affinity maturation. Science. 2007;5811:528–531. - PubMed
-
- Allman D, Jain A, Dent A, Maile RR, Selvaggi T, Kehry MR, Staudt LM. BCL-6 expression during B-cell activation. Blood. 1996;12:5257–5268. - PubMed
-
- Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–1129. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

