Modulation of in vitro murine B-lymphocyte response by curcumin
- PMID: 19303754
- PMCID: PMC2908300
- DOI: 10.1016/j.phymed.2009.01.004
Modulation of in vitro murine B-lymphocyte response by curcumin
Abstract
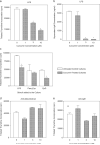
Curcumin is a phenolic natural product isolated from the rhizome of Curcuma longa (tumeric). It was previously described that curcumin had a potent anti-inflammatory effect and inhibited the proliferation of a variety of tumor cells. In the present study, we investigated the inhibitory effects of curcumin on the response of normal murine splenic B cells. Curcumin inhibited the proliferative response of purified splenic B cells from BALB/c mice stimulated with the Toll-like receptor ligands LPS and CpG oligodeoxynucleotides. LPS-induced IgM secretion was also inhibited by curcumin. The proliferative response induced by either the T-independent type 2 stimuli anti-delta-dextran or anti-IgM antibodies was relatively resistant to the effect of curcumin. We investigated the intracellular signaling events involved in the inhibitory effects of curcumin on murine B cells. Curcumin did not inhibit the increase in calcium levels induced by anti-IgM antibody. Western blotting analysis showed that curcumin inhibited TLR ligands and anti-IgM-induced phosphorylation of ERK, IkappaB and p38. Curcumin also decreased the nuclear levels of NFkappaB. Our results suggested that curcumin is an important inhibitor of signaling pathways activated upon B cell stimulation by TLR ligands. These data indicate that curcumin could be a potent pharmacological inhibitor of B cell activation.
Figures
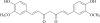

References
-
- Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006;71:1397–1421. - PubMed
-
- Benschop RJ, Aviszus K, Zhang X, Manser T, Cambier JC, Wysocki LJ. Activation and anergy in bone marrow B cells of a novel immunoglobulin transgenic mouse that is both hapten specific and autoreactive. Immunity. 2001;14:33–43. - PubMed
-
- Bijsterbosch MK, Meade CJ, Turner GA, Klaus GG. B lymphocyte receptors and polyphosphoinositide degradation. Cell. 1985;41:999–1006. - PubMed
-
- Brunswick M, Finkelman FD, Highet PF, Inman JK, Dintzis HM, Mond JJ. Picogram quantities of anti-Ig antibodies coupled to dextran induce B cell proliferation. J. Immunol. 1988;140:3364–3372. - PubMed
-
- Churchill M, Chadburn A, Bilinski RT, Bertagnolli MM. Inhibition of intestinal tumors by curcumin is associated with changes in the intestinal immune cell profile. J. Surg. Res. 2000;89:169–175. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

