Adoptive cell therapy for the treatment of patients with metastatic melanoma
- PMID: 19304471
- PMCID: PMC3459355
- DOI: 10.1016/j.coi.2009.03.002
Adoptive cell therapy for the treatment of patients with metastatic melanoma
Abstract
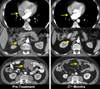
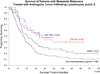
Adoptive cell therapy (ACT) is the best available treatment for patients with metastatic melanoma. In a recent series of three consecutive clinical trials using increasing lymphodepletion before infusion of autologous tumor infiltrating lymphocytes (TIL), objective response rates between 49% and 72% were seen. Persistence of infused cells in the circulation at one month was highly correlated with anti-tumor response as was the mean telomere length of the cells infused and the number of CD8+ CD27+ cells infused. Responses occur at all sites and appear to be durable with many patients in ongoing response beyond three years. In the most recent trial of 25 patients receiving maximum lymphodepletion, seven of the 25 patients (28%) achieved a complete response. Of the 12 patients in the three trials who achieved a complete response all but one are ongoing between 18 and 75 months. We recently demonstrated that ACT using autologous lymphocytes genetically modified to express anti-tumor T cell receptors can mediate tumor regression and this approach is now being applied to patients with common epithelial cancers.
Figures






References
-
- Muul LM, Spiess PJ, Director EP, Rosenberg SA. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J. Immunol. 1987;138:989–995. - PubMed
-
- Van der Bruggen P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643–1647. - PubMed
-
- Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–287. - PubMed
-
-
Dudley ME, et al. Adoptive cell therapy for patients with metastatic melanoma: Evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. Three lymphodepleting regimens were combined with tumor reactive TIL administration and compared for safety and efficacy in sequential ACT clinical trials. Overall 52 of 93 patients (56%) experienced an objective response
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

