Cost effectiveness analysis of larval therapy for leg ulcers
- PMID: 19304578
- PMCID: PMC2659856
- DOI: 10.1136/bmj.b825
Cost effectiveness analysis of larval therapy for leg ulcers
Abstract
Objective: To assess the cost effectiveness of larval therapy compared with hydrogel in the management of leg ulcers.
Design: Cost effectiveness and cost utility analyses carried out alongside a pragmatic multicentre, randomised, open trial with equal randomisation. Population Intention to treat population comprising 267 patients with a venous or mixed venous and arterial ulcers with at least 25% coverage of slough or necrotic tissue.
Interventions: Patients were randomly allocated to debridement with bagged larvae, loose larvae, or hydrogel.
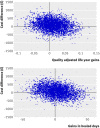
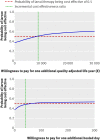
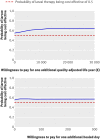
Main outcome measure: The time horizon was 12 months and costs were estimated from the UK National Health Service perspective. Cost effectiveness outcomes are expressed in terms of incremental costs per ulcer-free day (cost effectiveness analysis) and incremental costs per quality adjusted life years (cost utility analysis).
Results: The larvae arms were pooled for the main analysis. Treatment with larval therapy cost, on average, pound96.70 (euro109.61; $140.57) more per participant per year (95% confidence interval - pound491.9 to pound685.8) than treatment with hydrogel. Participants treated with larval therapy healed, on average, 2.42 days before those in the hydrogel arm (95% confidence interval -0.95 to 31.91 days) and had a slightly better health related quality of life, as the annual difference in QALYs was 0.011 (95% confidence interval -0.067 to 0.071). However, none of these differences was statistically significant. The incremental cost effectiveness ratio for the base case analysis was estimated at pound8826 per QALY gained and pound40 per ulcer-free day. Considerable uncertainty surrounds the outcome estimates.
Conclusions: Debridement of sloughy or necrotic leg ulcers with larval therapy is likely to produce similar health benefits and have similar costs to treatment with hydrogel.
Trial registration: Current Controlled Trials ISRCTN55114812 and National Research Register N0484123692.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Nelzen O, Bergqvist D, Lindhagen A. Venous and non-venous leg ulcers: clinical history and appearance in a population study. Br J Surg 1994;81:182-7. - PubMed
-
- Cullum N, Nelson EA, Flemming K, Sheldon T. Systematic reviews of wound care management: (5) beds; (6) compression; (7) laser therapy, therapeutic ultrasound, electrotherapy and electromagnetic therapy. Health Technol Assess 2001;5:1-221. - PubMed
-
- Cullum N, Nelson EA, Fletcher AW, Sheldon TA. Compression for venous leg ulcers.[update of Cochrane Database Syst Rev 2000;(3):CD000265]. Cochrane Database Syst Rev 2001(2):CD000265. - PubMed
-
- Iglesias C, Nelson EA, Cullum NA, Torgerson DJ, Ven UST. VenUS I: a randomised controlled trial of two types of bandage for treating venous leg ulcers. Health Technol Assess 2004;8(29):iii. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical