Familial FTDP-17 missense mutations inhibit microtubule assembly-promoting activity of tau by increasing phosphorylation at Ser202 in vitro
- PMID: 19304664
- PMCID: PMC2679442
- DOI: 10.1074/jbc.M901095200
Familial FTDP-17 missense mutations inhibit microtubule assembly-promoting activity of tau by increasing phosphorylation at Ser202 in vitro
Abstract
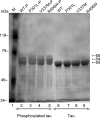
In Alzheimer disease (AD), frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17) and other tauopathies, tau accumulates and forms paired helical filaments (PHFs) in the brain. Tau isolated from PHFs is phosphorylated at a number of sites, migrates as approximately 60-, 64-, and 68-kDa bands on SDS-gel, and does not promote microtubule assembly. Upon dephosphorylation, the PHF-tau migrates as approximately 50-60-kDa bands on SDS-gels in a manner similar to tau that is isolated from normal brain and promotes microtubule assembly. The site(s) that inhibits microtubule assembly-promoting activity when phosphorylated in the diseased brain is not known. In this study, when tau was phosphorylated by Cdk5 in vitro, its mobility shifted from approximately 60-kDa bands to approximately 64- and 68-kDa bands in a time-dependent manner. This mobility shift correlated with phosphorylation at Ser(202), and Ser(202) phosphorylation inhibited tau microtubule-assembly promoting activity. When several tau point mutants were analyzed, G272V, P301L, V337M, and R406W mutations associated with FTDP-17, but not nonspecific mutations S214A and S262A, promoted Ser(202) phosphorylation and mobility shift to a approximately 68-kDa band. Furthermore, Ser(202) phosphorylation inhibited the microtubule assembly-promoting activity of FTDP-17 mutants more than of WT. Our data indicate that FTDP-17 missense mutations, by promoting phosphorylation at Ser(202), inhibit the microtubule assembly-promoting activity of tau in vitro, suggesting that Ser(202) phosphorylation plays a major role in the development of NFT pathology in AD and related tauopathies.
Figures

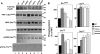







Similar articles
-
Effect of Pin1 or microtubule binding on dephosphorylation of FTDP-17 mutant Tau.J Biol Chem. 2009 Jun 19;284(25):16840-16847. doi: 10.1074/jbc.M109.003277. Epub 2009 Apr 28. J Biol Chem. 2009. PMID: 19401603 Free PMC article.
-
FTDP-17 missense mutations site-specifically inhibit as well as promote dephosphorylation of microtubule-associated protein tau by protein phosphatases of HEK-293 cell extract.Neurochem Int. 2009 Jan;54(1):14-27. doi: 10.1016/j.neuint.2008.09.014. Epub 2008 Oct 15. Neurochem Int. 2009. PMID: 18992292
-
Missense point mutations of tau to segregate with FTDP-17 exhibit site-specific effects on microtubule structure in COS cells: a novel action of R406W mutation.J Neurosci Res. 2000 May 1;60(3):380-7. doi: 10.1002/(SICI)1097-4547(20000501)60:3<380::AID-JNR13>3.0.CO;2-5. J Neurosci Res. 2000. PMID: 10797541
-
Frontotemporal dementia with tau pathology.Neurodegener Dis. 2007;4(2-3):236-53. doi: 10.1159/000101848. Neurodegener Dis. 2007. PMID: 17596718 Review.
-
Tau pathology in Alzheimer disease and other tauopathies.Biochim Biophys Acta. 2005 Jan 3;1739(2-3):198-210. doi: 10.1016/j.bbadis.2004.09.008. Biochim Biophys Acta. 2005. PMID: 15615638 Review.
Cited by
-
Stable mutated tau441 transfected SH-SY5Y cells as screening tool for Alzheimer's disease drug candidates.J Mol Neurosci. 2012 May;47(1):192-203. doi: 10.1007/s12031-012-9716-6. Epub 2012 Feb 19. J Mol Neurosci. 2012. PMID: 22351109 Free PMC article.
-
14-3-3ζ Mediates Tau Aggregation in Human Neuroblastoma M17 Cells.PLoS One. 2016 Aug 22;11(8):e0160635. doi: 10.1371/journal.pone.0160635. eCollection 2016. PLoS One. 2016. PMID: 27548710 Free PMC article.
-
Hypoxia alters expression of zebrafish microtubule-associated protein tau (mapta, maptb) gene transcripts.BMC Res Notes. 2014 Oct 31;7:767. doi: 10.1186/1756-0500-7-767. BMC Res Notes. 2014. PMID: 25359609 Free PMC article.
-
Protein phosphorylation in neurodegeneration: friend or foe?Front Mol Neurosci. 2014 May 13;7:42. doi: 10.3389/fnmol.2014.00042. eCollection 2014. Front Mol Neurosci. 2014. PMID: 24860424 Free PMC article. Review.
-
Chaperone-dependent Neurodegeneration: A Molecular Perspective on Therapeutic Intervention.J Alzheimers Dis Parkinsonism. 2013 Apr;2013(Suppl 10):007. doi: 10.4172/2161-0460.S10-007. J Alzheimers Dis Parkinsonism. 2013. PMID: 25258700 Free PMC article.
References
-
- Iqbal, K., Alonso Adel, C., Chen, S., Chohan, M. O., El-Akkad, E., Gong, C. X., Khatoon, S., Li, B., Liu, F., Rahman, A., Tanimukai, H., and Grundke-Iqbal, I. (2005) Biochim. Biophys. Acta 1739 198–210 - PubMed
-
- Lee, V. M., Goedert, M., and Trojanowski, J. Q. (2001) Annu. Rev. Neurosci. 24 1121–1159 - PubMed
-
- Avila, J., Lucas, J. J., Perez, M., and Hernandez, F. (2004) Physiol. Rev. 84 361–384 - PubMed
-
- Lee, V. M., Balin, B. J., Otvos, L., Jr., and Trojanowski, J. Q. (1991) Science 251 675–678 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

