Construction of a gene knockout system for application in Paenibacillus alvei CCM 2051T, exemplified by the S-layer glycan biosynthesis initiation enzyme WsfP
- PMID: 19304819
- PMCID: PMC2681630
- DOI: 10.1128/AEM.00087-09
Construction of a gene knockout system for application in Paenibacillus alvei CCM 2051T, exemplified by the S-layer glycan biosynthesis initiation enzyme WsfP
Abstract
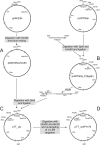
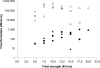
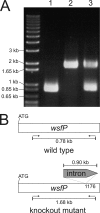
The gram-positive bacterium Paenibacillus alvei CCM 2051T is covered by an oblique surface layer (S-layer) composed of glycoprotein subunits. The S-layer O-glycan is a polymer of [-->3)-beta-D-Galp-(1[alpha-D-Glcp-(1-->6)]-->4)-beta-D-ManpNAc-(1-->] repeating units that is linked by an adaptor of -[GroA-2-->OPO2-->4-beta-D-ManpNAc-(1-->4)]-->3)-alpha-L-Rhap-(1-->3)-alpha-L-Rhap-(1-->3)-alpha-L-Rhap-(1-->3)-beta-D-Galp-(1--> to specific tyrosine residues of the S-layer protein. For elucidation of the mechanism governing S-layer glycan biosynthesis, a gene knockout system using bacterial mobile group II intron-mediated gene disruption was developed. The system is further based on the sgsE S-layer gene promoter of Geobacillus stearothermophilus NRS 2004/3a and on the Geobacillus-Bacillus-Escherichia coli shuttle vector pNW33N. As a target gene, wsfP, encoding a putative UDP-Gal:phosphoryl-polyprenol Gal-1-phosphate transferase, representing the predicted initiation enzyme of S-layer glycan biosynthesis, was disrupted. S-layer protein glycosylation was completely abolished in the insertional P. alvei CCM 2051T wsfP mutant, according to sodium dodecyl sulfate-polyacrylamide gel electrophoresis evidence and carbohydrate analysis. Glycosylation was fully restored by plasmid-based expression of wsfP in the glycan-deficient P. alvei mutant, confirming that WsfP initiates S-layer protein glycosylation. This is the first report on the successful genetic manipulation of bacterial S-layer protein glycosylation in vivo, including transformation of and heterologous gene expression and gene disruption in the model organism P. alvei CCM 2051T.
Figures

Similar articles
-
Protein tyrosine O-glycosylation--a rather unexplored prokaryotic glycosylation system.Glycobiology. 2010 Jun;20(6):787-98. doi: 10.1093/glycob/cwq035. Epub 2010 Mar 3. Glycobiology. 2010. PMID: 20200052 Free PMC article.
-
Molecular basis of S-layer glycoprotein glycan biosynthesis in Geobacillus stearothermophilus.J Biol Chem. 2008 Jul 25;283(30):21120-33. doi: 10.1074/jbc.M801833200. Epub 2008 May 30. J Biol Chem. 2008. PMID: 18515358 Free PMC article.
-
Functional characterization of the initiation enzyme of S-layer glycoprotein glycan biosynthesis in Geobacillus stearothermophilus NRS 2004/3a.J Bacteriol. 2007 Apr;189(7):2590-8. doi: 10.1128/JB.01592-06. Epub 2007 Jan 19. J Bacteriol. 2007. PMID: 17237178 Free PMC article.
-
Genetic organization of chromosomal S-layer glycan biosynthesis loci of Bacillaceae.Glycoconj J. 2004;20(7-8):435-47. doi: 10.1023/B:GLYC.0000038290.74944.65. Glycoconj J. 2004. PMID: 15316277 Review.
-
S-layer nanoglycobiology of bacteria.Carbohydr Res. 2008 Aug 11;343(12):1934-51. doi: 10.1016/j.carres.2007.12.025. Epub 2008 Jan 16. Carbohydr Res. 2008. PMID: 18336801 Free PMC article. Review.
Cited by
-
The brown algae Pl.LSU/2 group II intron-encoded protein has functional reverse transcriptase and maturase activities.PLoS One. 2013;8(3):e58263. doi: 10.1371/journal.pone.0058263. Epub 2013 Mar 11. PLoS One. 2013. PMID: 23505475 Free PMC article.
-
Use of RmInt1, a group IIB intron lacking the intron-encoded protein endonuclease domain, in gene targeting.Appl Environ Microbiol. 2011 Feb;77(3):854-61. doi: 10.1128/AEM.02319-10. Epub 2010 Nov 29. Appl Environ Microbiol. 2011. PMID: 21115708 Free PMC article.
-
Swarming motility and biofilm formation of Paenibacillus larvae, the etiological agent of American Foulbrood of honey bees (Apis mellifera).Sci Rep. 2018 Jun 11;8(1):8840. doi: 10.1038/s41598-018-27193-8. Sci Rep. 2018. PMID: 29892084 Free PMC article.
-
CRISPR-Cas9-Mediated Genome Editing in Paenibacillus polymyxa.Methods Mol Biol. 2024;2760:267-280. doi: 10.1007/978-1-0716-3658-9_16. Methods Mol Biol. 2024. PMID: 38468094
-
Biotechnological applications of mobile group II introns and their reverse transcriptases: gene targeting, RNA-seq, and non-coding RNA analysis.Mob DNA. 2014 Jan 13;5(1):2. doi: 10.1186/1759-8753-5-2. Mob DNA. 2014. PMID: 24410776 Free PMC article.
References
-
- Abu-Qarn, M., J. Eichler, and N. Sharon. 2008. Not just for Eukarya anymore: protein glycosylation in Bacteria and Archaea. Curr. Opin. Struct. Biol. 18:544-550. - PubMed
-
- Altman, E., J. R. Brisson, P. Messner, and U. B. Sleytr. 1991. Structure of the glycan chain from the surface layer glycoprotein of Bacillus alvei CCM 2051. Biochem. Cell Biol. 69:72-78. - PubMed
-
- Altman, E., C. Schäffer, J.-R. Brisson, and P. Messner. 1995. Characterization of the glycan structure of a major glycopeptide from the surface layer glycoprotein of Clostridium thermosaccharolyticum E207-71. Eur. J. Biochem. 229:308-315. - PubMed
-
- Ash, C., F. G. Priest, and M. D. Collins. 1993. Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie van Leeuwenhoek 64:253-260. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

