The glutathione/glutaredoxin system is essential for arsenate reduction in Synechocystis sp. strain PCC 6803
- PMID: 19304854
- PMCID: PMC2681892
- DOI: 10.1128/JB.01798-08
The glutathione/glutaredoxin system is essential for arsenate reduction in Synechocystis sp. strain PCC 6803
Abstract
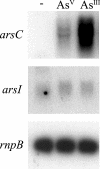
Arsenic resistance in Synechocystis sp. strain PCC 6803 is mediated by an operon of three genes in which arsC codes for an arsenate reductase with unique characteristics. Here we describe the identification of two additional and nearly identical genes coding for arsenate reductases in Synechocystis sp. strain PCC 6803, which we have designed arsI1 and arsI2, and the biochemical characterization of both ArsC (arsenate reductase) and ArsI. Functional analysis of single, double, and triple mutants shows that both ArsI enzymes are active arsenate reductases but that their roles in arsenate resistance are essential only in the absence of ArsC. Based on its biochemical properties, ArsC belongs to a family that, though related to thioredoxin-dependent arsenate reductases, uses the glutathione/glutaredoxin system for reduction, whereas ArsI belongs to the previously known glutaredoxin-dependent family. We have also analyzed the role in arsenate resistance of the three glutaredoxins present in Synechocystis sp. strain PCC 6803 both in vitro and in vivo. Only the dithiolic glutaredoxins, GrxA (glutaredoxin A) and GrxB (glutaredoxin B), are able to donate electrons to both types of reductases in vitro, while GrxC (glutaredoxin C), a monothiolic glutaredoxin, is unable to donate electrons to either type. Analysis of glutaredoxin mutant strains revealed that only those lacking the grxA gene have impaired arsenic resistance.
Figures




Similar articles
-
A Hybrid Mechanism for the Synechocystis Arsenate Reductase Revealed by Structural Snapshots during Arsenate Reduction.J Biol Chem. 2015 Sep 4;290(36):22262-73. doi: 10.1074/jbc.M115.659896. Epub 2015 Jul 29. J Biol Chem. 2015. PMID: 26224634 Free PMC article.
-
Redox, mutagenic and structural studies of the glutaredoxin/arsenate reductase couple from the cyanobacterium Synechocystis sp. PCC 6803.Biochim Biophys Acta. 2012 Feb;1824(2):392-403. doi: 10.1016/j.bbapap.2011.10.012. Epub 2011 Oct 29. Biochim Biophys Acta. 2012. PMID: 22155275
-
1H, 13C and 15N resonance assignments of the arsenate reductase from Synechocystis sp. strain PCC 6803.Biomol NMR Assign. 2011 Apr;5(1):85-7. doi: 10.1007/s12104-010-9273-2. Epub 2010 Oct 20. Biomol NMR Assign. 2011. PMID: 20960080
-
Arsenate reduction: thiol cascade chemistry with convergent evolution.J Mol Biol. 2006 Sep 8;362(1):1-17. doi: 10.1016/j.jmb.2006.07.002. Epub 2006 Aug 14. J Mol Biol. 2006. PMID: 16905151 Review.
-
Genetic identification of arsenate reductase and arsenite oxidase in redox transformations carried out by arsenic metabolising prokaryotes - A comprehensive review.Chemosphere. 2016 Nov;163:400-412. doi: 10.1016/j.chemosphere.2016.08.044. Epub 2016 Aug 24. Chemosphere. 2016. PMID: 27565307 Review.
Cited by
-
Regulatory Activities of Four ArsR Proteins in Agrobacterium tumefaciens 5A.Appl Environ Microbiol. 2016 May 31;82(12):3471-3480. doi: 10.1128/AEM.00262-16. Print 2016 Jun 15. Appl Environ Microbiol. 2016. PMID: 27037117 Free PMC article.
-
Whole Genome Sequence Analysis of Cupriavidus necator C39, a Multiple Heavy Metal(loid) and Antibiotic Resistant Bacterium Isolated from a Gold/Copper Mine.Microorganisms. 2023 Jun 7;11(6):1518. doi: 10.3390/microorganisms11061518. Microorganisms. 2023. PMID: 37375020 Free PMC article.
-
A new arsenate reductase involved in arsenic detoxification in Anabaena sp. PCC7120.Funct Integr Genomics. 2013 Mar;13(1):43-55. doi: 10.1007/s10142-012-0296-x. Epub 2012 Oct 21. Funct Integr Genomics. 2013. PMID: 23086594
-
Deciphering the mechanism of glutaredoxin-catalyzed roGFP2 redox sensing reveals a ternary complex with glutathione for protein disulfide reduction.Nat Commun. 2024 Feb 26;15(1):1733. doi: 10.1038/s41467-024-45808-9. Nat Commun. 2024. PMID: 38409212 Free PMC article.
-
Essential role of glutathione in acclimation to environmental and redox perturbations in the cyanobacterium Synechocystis sp. PCC 6803.Plant Physiol. 2010 Dec;154(4):1672-85. doi: 10.1104/pp.110.162990. Epub 2010 Oct 8. Plant Physiol. 2010. PMID: 20935175 Free PMC article.
References
-
- Bandyopadhyay, S., F. Gama, M. M. Molina-Navarro, J. M. Gualberto, R. Claxton, S. G. Naik, B. H. Huynh, E. Herrero, J. P. Jacquot, M. K. Johnson, and N. Rouhier. 2008. Chloroplast monothiol glutaredoxins as scaffold proteins for the assembly and delivery of [2Fe-2S] clusters. EMBO J. 271122-1133. - PMC - PubMed
-
- Cánovas, D., I. Cases, and V. de Lorenzo. 2003. Heavy metal tolerance and metal homeostasis in Pseudomonas putida as revealed by complete genome analysis. Environ. Microbiol. 51242-1256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

