Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation
- PMID: 19304887
- PMCID: PMC2687466
- DOI: 10.1242/dev.027342
Recruitment and maintenance of tendon progenitors by TGFbeta signaling are essential for tendon formation
Abstract
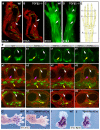
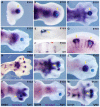
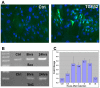
Tendons and ligaments mediate the attachment of muscle to bone and of bone to bone to provide connectivity and structural integrity in the musculoskeletal system. We show that TGFbeta signaling plays a major role in the formation of these tissues. TGFbeta signaling is a potent inducer of the tendon progenitor (TNP) marker scleraxis both in organ culture and in cultured cells, and disruption of TGFbeta signaling in Tgfb2(-/-);Tgfb3(-/-) double mutant embryos or through inactivation of the type II TGFbeta receptor (TGFBR2; also known as TbetaRII) results in the loss of most tendons and ligaments in the limbs, trunk, tail and head. The induction of scleraxis-expressing TNPs is not affected in mutant embryos and the tendon phenotype is first manifested at E12.5, a developmental stage in which TNPs are positioned between the differentiating muscles and cartilage, and in which Tgfb2 or Tgfb3 is expressed both in TNPs and in the differentiating muscles and cartilage. TGFbeta signaling is thus essential for maintenance of TNPs, and we propose that it also mediates the recruitment of new tendon cells by differentiating muscles and cartilage to establish the connections between tendon primordia and their respective musculoskeletal counterparts, leading to the formation of an interconnected and functionally integrated musculoskeletal system.
Figures






References
-
- Anderson, D. M., Arredondo, J., Hahn, K., Valente, G., Martin, J. F., Wilson-Rawls, J. and Rawls, A. (2006). Mohawk is a novel homeobox gene expressed in the developing mouse embryo. Dev. Dyn. 235, 792-801. - PubMed
-
- Baffi, M. O., Slattery, E., Sohn, P., Moses, H. L., Chytil, A. and Serra, R. (2004). Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev. Biol. 276, 124-142. - PubMed
-
- Bi, Y., Ehirchiou, D., Kilts, T. M., Inkson, C. A., Embree, M. C., Sonoyama, W., Li, L., Leet, A. I., Seo, B. M., Zhang, L. et al. (2007). Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 13, 1219-1227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

