Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis
- PMID: 19304910
- PMCID: PMC2692804
- DOI: 10.1152/ajplung.90512.2008
Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis
Abstract
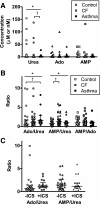
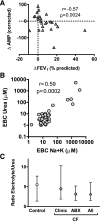
Exhaled breath condensate (EBC) analyses promise simple and noninvasive methods to measure airway biomarkers but pose considerable methodological challenges. We utilized mass spectrometry to measure EBC purine biomarkers adenosine and AMP plus urea to control for dilutional variability in two studies: 1) a cross-sectional analysis of 28 healthy, 40 cystic fibrosis (CF), and 11 asthmatic children; and 2) a longitudinal analysis of 26 CF children before and after treatment of a pulmonary exacerbation. EBC adenosine, AMP, and urea were readily detected and quantified by mass spectrometry, and analysis suggested significant dilutional variability. Using biomarker-to-urea ratios to control for dilution, the EBC AMP-to-urea ratio was elevated in CF [median 1.3, interquartile range (IQR) 0.7-2.3] vs. control (median 0.75, IQR 0.3-1.4; P < 0.05), and the adenosine-to-urea ratio was elevated in asthma (median 1.5, IQR 0.9-2.9) vs. control (median 0.4, IQR 0.2-1.6; P < 0.05). Changes in EBC purine-to-urea ratios correlated with changes in percent predicted forced expiratory volume in 1 s (FEV(1)) (r = -0.53 AMP/urea, r = -0.55 adenosine/urea; P < 0.01 for both) after CF exacerbation treatment. Similar results were observed using dilution factors calculated from serum-to-EBC urea ratios or EBC electrolytes, and the comparable ratios of EBC electrolytes to urea in CF and control (median 3.2, IQR 1.6-6.0 CF; median 5.5, IQR 1.4-7.7 control) validated use of airway urea as an EBC dilution marker. These results show that mass spectrometric analyses can be applied to measurement of purines in EBC and demonstrate that EBC adenosine-to-urea and AMP-to-urea ratios are potential noninvasive biomarkers of airways disease.
Figures


Similar articles
-
Exhaled breath condensate adenosine tracks lung function changes in cystic fibrosis.Am J Physiol Lung Cell Mol Physiol. 2013 Apr 1;304(7):L504-9. doi: 10.1152/ajplung.00344.2012. Epub 2013 Jan 25. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23355385 Free PMC article. Clinical Trial.
-
A mass spectrometric method to simultaneously measure a biomarker and dilution marker in exhaled breath condensate.Rapid Commun Mass Spectrom. 2008;22(5):701-5. doi: 10.1002/rcm.3408. Rapid Commun Mass Spectrom. 2008. PMID: 18257110 Free PMC article.
-
Exhaled breath condensate purines correlate with lung function in infants and preschoolers.Pediatr Pulmonol. 2013 Feb;48(2):182-7. doi: 10.1002/ppul.22573. Epub 2012 May 21. Pediatr Pulmonol. 2013. PMID: 22615171 Free PMC article.
-
Clinical metabolomics of exhaled breath condensate in chronic respiratory diseases.Adv Clin Chem. 2019;88:121-149. doi: 10.1016/bs.acc.2018.10.002. Epub 2018 Nov 22. Adv Clin Chem. 2019. PMID: 30612604 Review.
-
Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease.J Allergy Clin Immunol. 2002 Jul;110(1):28-34. doi: 10.1067/mai.2002.124966. J Allergy Clin Immunol. 2002. PMID: 12110814 Review.
Cited by
-
Exhaled breath condensate adenosine tracks lung function changes in cystic fibrosis.Am J Physiol Lung Cell Mol Physiol. 2013 Apr 1;304(7):L504-9. doi: 10.1152/ajplung.00344.2012. Epub 2013 Jan 25. Am J Physiol Lung Cell Mol Physiol. 2013. PMID: 23355385 Free PMC article. Clinical Trial.
-
Effects of bronchoconstriction, minute ventilation, and deep inspiration on the composition of exhaled breath condensate.Chest. 2011 Jan;139(1):16-22. doi: 10.1378/chest.10-0101. Epub 2010 Apr 9. Chest. 2011. PMID: 20382713 Free PMC article.
-
Future directions in early cystic fibrosis lung disease research: an NHLBI workshop report.Am J Respir Crit Care Med. 2012 Apr 15;185(8):887-92. doi: 10.1164/rccm.201111-2068WS. Epub 2012 Feb 3. Am J Respir Crit Care Med. 2012. PMID: 22312017 Free PMC article.
-
Loss of β Epithelial Sodium Channel Function in Meibomian Glands Produces Pseudohypoaldosteronism 1-Like Ocular Disease in Mice.Am J Pathol. 2018 Jan;188(1):95-110. doi: 10.1016/j.ajpath.2017.09.016. Epub 2017 Oct 26. Am J Pathol. 2018. PMID: 29107074 Free PMC article.
-
Metabolomics in Childhood Asthma - a Promising Tool to Meet Various Clinical Needs.Curr Allergy Asthma Rep. 2025 May 9;25(1):24. doi: 10.1007/s11882-025-01198-6. Curr Allergy Asthma Rep. 2025. PMID: 40341431 Free PMC article. Review.
References
-
- Bloemen K, Lissens G, Desager K, Schoeters G. Determinants of variability of protein content, volume and pH of exhaled breath condensate. Respir Med 101: 1331–1337, 2007. - PubMed
-
- Bodini A, D'Orazio C, Peroni D, Corradi M, Folesani G, Baraldi E, Assael BM, Boner A, Piacentini GL. Biomarkers of neutrophilic inflammation in exhaled air of cystic fibrosis children with bacterial airway infections. Pediatr Pulmonol 40: 494–499, 2005. - PubMed
-
- Burnstock G Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 58: 58–86, 2006. - PubMed
-
- Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795, 2006. - PubMed

