Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone
- PMID: 19304912
- PMCID: PMC3286237
- DOI: 10.1152/ajplung.90392.2008
Hyperoxia-induced neonatal rat lung injury involves activation of TGF-{beta} and Wnt signaling and is protected by rosiglitazone
Abstract
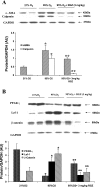
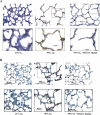
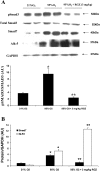
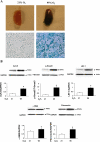
Despite tremendous technological and therapeutic advances, bronchopulmonary dysplasia (BPD) remains a leading cause of respiratory morbidity in very low birth weight infants, and there are no effective preventive and/or therapeutic options. We have previously reported that hyperoxia-induced neonatal rat lung injury might be prevented by rosiglitazone (RGZ). Here, we characterize 1) perturbations in wingless/Int (Wnt) and transforming growth factor (TGF)-beta signaling, and 2) structural aberrations in lung morphology following 7-day continuous in vivo hyperoxia exposure to neonatal rats. We also tested whether treatment of neonatal pups with RGZ, concomitant to hyperoxia, could prevent such aberrations. Our study revealed that hyperoxia caused significant upregulation of Wnt signaling protein markers lymphoid enhancer factor 1 (Lef-1) and beta-catenin and TGF-beta pathway transducers phosphorylated Smad3 and Smad7 proteins in whole rat lung extracts. These changes were also accompanied by upregulation of myogenic marker proteins alpha-smooth muscle actin (alpha-SMA) and calponin but significant downregulation of the lipogenic marker peroxisome proliferator-activated receptor-gamma (PPARgamma) expression. These molecular perturbations were associated with reduction in alveolar septal thickness, radial alveolar count, and larger alveoli in the hyperoxia-exposed lung. These hyperoxia-induced molecular and morphological changes were prevented by systemic administration of RGZ, with lung sections appearing near normal. This is the first evidence that in vivo hyperoxia induces activation of both Wnt and TGF-beta signal transduction pathways in lung and of its near complete prevention by RGZ. Hyperoxia-induced arrest in alveolar development, a hallmark of BPD, along with these molecular changes strongly implicates these proteins in hyperoxia-induced lung injury. Administration of PPARgamma agonists may thus be a potential strategy to attenuate hyperoxia-induced lung injury and subsequent BPD.
Figures


Similar articles
-
Antenatally administered PPAR-gamma agonist rosiglitazone prevents hyperoxia-induced neonatal rat lung injury.Am J Physiol Lung Cell Mol Physiol. 2010 Nov;299(5):L672-80. doi: 10.1152/ajplung.00240.2010. Epub 2010 Aug 20. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20729387 Free PMC article.
-
Rosiglitazone, a peroxisome proliferator-activated receptor-gamma agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo.Pediatr Pulmonol. 2006 Jun;41(6):558-69. doi: 10.1002/ppul.20407. Pediatr Pulmonol. 2006. PMID: 16617452
-
Curcumin augments lung maturation, preventing neonatal lung injury by inhibiting TGF-β signaling.Am J Physiol Lung Cell Mol Physiol. 2011 Nov;301(5):L721-30. doi: 10.1152/ajplung.00076.2011. Epub 2011 Aug 5. Am J Physiol Lung Cell Mol Physiol. 2011. PMID: 21821729 Free PMC article.
-
Bronchopulmonary Dysplasia: Crosstalk Between PPARγ, WNT/β-Catenin and TGF-β Pathways; The Potential Therapeutic Role of PPARγ Agonists.Front Pediatr. 2019 May 3;7:176. doi: 10.3389/fped.2019.00176. eCollection 2019. Front Pediatr. 2019. PMID: 31131268 Free PMC article. Review.
-
The Interplay of WNT and PPARγ Signaling in Vascular Calcification.Cells. 2020 Dec 10;9(12):2658. doi: 10.3390/cells9122658. Cells. 2020. PMID: 33322009 Free PMC article. Review.
Cited by
-
Sex-specific perinatal nicotine-induced asthma in rat offspring.Am J Respir Cell Mol Biol. 2013 Jan;48(1):53-62. doi: 10.1165/rcmb.2011-0344OC. Epub 2012 Sep 20. Am J Respir Cell Mol Biol. 2013. PMID: 23002101 Free PMC article.
-
MicroRNA-mRNA interactions in a murine model of hyperoxia-induced bronchopulmonary dysplasia.BMC Genomics. 2012 May 30;13:204. doi: 10.1186/1471-2164-13-204. BMC Genomics. 2012. PMID: 22646479 Free PMC article.
-
Modulators of inflammation in Bronchopulmonary Dysplasia.Semin Perinatol. 2018 Nov;42(7):459-470. doi: 10.1053/j.semperi.2018.09.009. Epub 2018 Oct 2. Semin Perinatol. 2018. PMID: 30446300 Free PMC article. Review.
-
Antenatally administered PPAR-gamma agonist rosiglitazone prevents hyperoxia-induced neonatal rat lung injury.Am J Physiol Lung Cell Mol Physiol. 2010 Nov;299(5):L672-80. doi: 10.1152/ajplung.00240.2010. Epub 2010 Aug 20. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20729387 Free PMC article.
-
Effects of hyperoxia on transdifferentiation of primary cultured typeII alveolar epithelial cells from premature rats.In Vitro Cell Dev Biol Anim. 2011 Jan;47(1):64-72. doi: 10.1007/s11626-010-9360-9. Epub 2010 Nov 17. In Vitro Cell Dev Biol Anim. 2011. PMID: 21082284
References
-
- Adamson IY, Young L, King GM. Reciprocal epithelial: fibroblast interactions in the control of fetal and adult rat lung cells in culture. Exp Lung Res 17: 821–835, 1991. - PubMed
-
- Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. - PubMed
-
- Bixby CE, Ibe BO, Abdallah MF, Zhou W, Hislop AA, Longo LD, Raj JU. Role of platelet activating factor in pulmonary vascular remodeling associated with chronic high altitude hypoxia in ovine fetal lambs. Am J Physiol Lung Cell Mol Physiol 293: L1475–L1482, 2007. - PubMed
-
- Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol 173: 2099–2108, 2004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

