Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection
- PMID: 19305394
- PMCID: PMC3885169
- DOI: 10.1038/ni.1720
Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection
Abstract
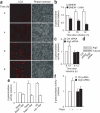
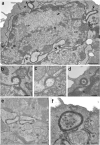
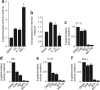
Viral proteins are usually processed by the 'classical' major histocompatibility complex (MHC) class I presentation pathway. Here we showed that although macrophages infected with herpes simplex virus type 1 (HSV-1) initially stimulated CD8(+) T cells by this pathway, a second pathway involving a vacuolar compartment was triggered later during infection. Morphological and functional analyses indicated that distinct forms of autophagy facilitated the presentation of HSV-1 antigens on MHC class I molecules. One form of autophagy involved a previously unknown type of autophagosome that originated from the nuclear envelope. Whereas interferon-gamma stimulated classical MHC class I presentation, fever-like hyperthermia and the pyrogenic cytokine interleukin 1beta activated autophagy and the vacuolar processing of viral peptides. Viral peptides in autophagosomes were further processed by the proteasome, which suggests a complex interaction between the vacuolar and MHC class I presentation pathways.
Figures



References
-
- Deretic V. Autophagy in innate and adaptive immunity. Trends Immunol. 2005;26:523–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

