Sperm from hyh mice carrying a point mutation in alphaSNAP have a defect in acrosome reaction
- PMID: 19305511
- PMCID: PMC2655651
- DOI: 10.1371/journal.pone.0004963
Sperm from hyh mice carrying a point mutation in alphaSNAP have a defect in acrosome reaction
Abstract
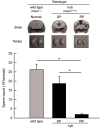
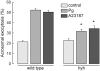
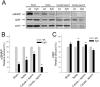
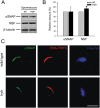
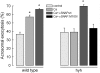
Hydrocephalus with hop gait (hyh) is a recessive inheritable disease that arose spontaneously in a mouse strain. A missense mutation in the Napa gene that results in the substitution of a methionine for isoleucine at position 105 (M105I) of alphaSNAP has been detected in these animals. alphaSNAP is a ubiquitous protein that plays a key role in membrane fusion and exocytosis. In this study, we found that male hyh mice with a mild phenotype produced morphologically normal and motile sperm, but had a strongly reduced fertility. When stimulated with progesterone or A23187 (a calcium ionophore), sperm from these animals had a defective acrosome reaction. It has been reported that the M105I mutation affects the expression but not the function of the protein. Consistent with an hypomorphic phenotype, the testes and epididymides of hyh mice had low amounts of the mutated protein. In contrast, sperm had alphaSNAP levels indistinguishable from those found in wild type cells, suggesting that the mutated protein is not fully functional for acrosomal exocytosis. Corroborating this possibility, addition of recombinant wild type alphaSNAP rescued exocytosis in streptolysin O-permeabilized sperm, while the mutant protein was ineffective. Moreover, addition of recombinant alphaSNAP. M105I inhibited acrosomal exocytosis in permeabilized human and wild type mouse sperm. We conclude that the M105I mutation affects the expression and also the function of alphaSNAP, and that a fully functional alphaSNAP is necessary for acrosomal exocytosis, a key event in fertilization.
Conflict of interest statement
Figures

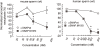
Similar articles
-
α-SNAP prevents docking of the acrosome during sperm exocytosis because it sequesters monomeric syntaxin.PLoS One. 2011;6(7):e21925. doi: 10.1371/journal.pone.0021925. Epub 2011 Jul 18. PLoS One. 2011. PMID: 21789195 Free PMC article.
-
Pleiotropic effects of alpha-SNAP M105I mutation on oocyte biology: ultrastructural and cellular changes that adversely affect female fertility in mice.Sci Rep. 2019 Nov 22;9(1):17374. doi: 10.1038/s41598-019-53574-8. Sci Rep. 2019. PMID: 31758001 Free PMC article.
-
Characterization of a novel role for the dynamin mechanoenzymes in the regulation of human sperm acrosomal exocytosis.Mol Hum Reprod. 2017 Oct 1;23(10):657-673. doi: 10.1093/molehr/gax044. Mol Hum Reprod. 2017. PMID: 29044420
-
Acrosomal exocytosis, a special type of regulated secretion.IUBMB Life. 2007 Apr-May;59(4-5):286-92. doi: 10.1080/15216540701222872. IUBMB Life. 2007. PMID: 17505967 Review.
-
Seeing is believing: Current methods to observe sperm acrosomal exocytosis in real time.Mol Reprod Dev. 2020 Dec;87(12):1188-1198. doi: 10.1002/mrd.23431. Epub 2020 Oct 28. Mol Reprod Dev. 2020. PMID: 33118273 Review.
Cited by
-
A framework for high-resolution phenotyping of candidate male infertility mutants: from human to mouse.Hum Genet. 2021 Jan;140(1):155-182. doi: 10.1007/s00439-020-02159-x. Epub 2020 Apr 4. Hum Genet. 2021. PMID: 32248361 Free PMC article. Review.
-
A vesicle trafficking protein αSNAP regulates Paneth cell differentiation in vivo.Biochem Biophys Res Commun. 2017 May 13;486(4):951-957. doi: 10.1016/j.bbrc.2017.03.135. Epub 2017 Mar 28. Biochem Biophys Res Commun. 2017. PMID: 28359759 Free PMC article.
-
Obesity significantly alters the human sperm proteome, with potential implications for fertility.J Assist Reprod Genet. 2020 Apr;37(4):777-787. doi: 10.1007/s10815-020-01707-8. Epub 2020 Feb 5. J Assist Reprod Genet. 2020. PMID: 32026202 Free PMC article.
-
Abnormal Transcytosis Mechanisms in the Pathogenesis of Hydrocephalus: A Review.Int J Mol Sci. 2025 May 19;26(10):4881. doi: 10.3390/ijms26104881. Int J Mol Sci. 2025. PMID: 40430021 Free PMC article. Review.
-
Rab3A, a possible marker of cortical granules, participates in cortical granule exocytosis in mouse eggs.Exp Cell Res. 2016 Sep 10;347(1):42-51. doi: 10.1016/j.yexcr.2016.07.005. Epub 2016 Jul 14. Exp Cell Res. 2016. PMID: 27423421 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

