GABA(A) receptors in normal development and seizures: friends or foes?
- PMID: 19305785
- PMCID: PMC2645547
- DOI: 10.2174/157015908783769653
GABA(A) receptors in normal development and seizures: friends or foes?
Abstract
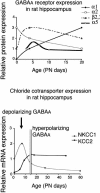
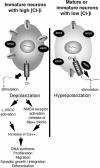
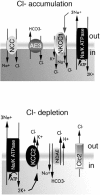
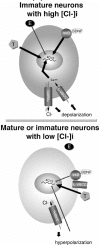
GABA(A) receptors have an age-adapted function in the brain. During early development, they mediate excitatory effects resulting in activation of calcium sensitive signaling processes that are important for the differentiation of the brain. In more mature stages of development and in adults, GABA(A) receptors transmit inhibitory signals. The maturation of GABA(A) signaling follows sex-specific patterns, which appear to also be important for the sexual differentiation of the brain. The inhibitory effects of GABA(A) receptor activation have been widely exploited in the treatment of conditions where neuronal silencing is necessary. For instance, drugs that target GABA(A) receptors are the mainstay of treatment of seizures. Recent evidence suggests however that the physiology and function of GABA(A) receptors changes in the brain of a subject that has epilepsy or status epilepticus.This review will summarize the physiology of and the developmental factors regulating the signaling and function of GABA(A) receptors; how these may change in the brain that has experienced prior seizures; what are the implications for the age and sex specific treatment of seizures and status epilepticus. Finally, the implications of these changes for the treatment of certain forms of medically refractory epilepsies and status epilepticus will be discussed.
Keywords: GABA; brain; chloride; development; expression; hippocampus; physiology.; seizure.
Figures

References
-
- Adelson PD, Black PM, Madsen JR, Kramer U, Rockoff MA, Riviello JJ, Helmers SL, Mikati M, Holmes GL. Use of subdural grids and strip electrodes to identify a seizure focus in children. Pediatr. Neurosurg. 1995;22(4):174–80. - PubMed
-
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB. Alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J. Biol. Chem. 2001;276(42):38934–9. - PubMed
-
- Aguado F, Carmona MA, Pozas E, Aguilo A, Martinez-Guijarro FJ, Alcantara S, Borrell V, Yuste R, Ibanez CF, Soriano E. BDNF regulates spontaneous correlated activity at early developmental stages by increasing synaptogenesis and expression of the K+/Cl- co-transporter KCC2. Development. 2003;130(7):1267–80. - PubMed
-
- Aickin CC, Deisz RA, Lux HD. Mechanisms of chloride transport in crayfish stretch receptor neurones and guinea pig vas deferens: implications for inhibition mediated by GABA. Neurosci. Lett. 1984;47(3):239–44. - PubMed
-
- Akaike N, Inomata N, Yakushiji T. Differential effects of extra- and intracellular anions on GABA-activated currents in bullfrog sensory neurons. J. Neurophysiol. 1989;62(6):1388–99. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
