Protein degradation in Parkinson disease revisited: it's complex
- PMID: 19306499
- PMCID: PMC2648670
- DOI: 10.1172/jci38619
Protein degradation in Parkinson disease revisited: it's complex
Abstract
Mutations in the genes PTEN-induced putative kinase 1 (PINK1), PARKIN,and DJ-1 cause autosomal recessive forms of Parkinson disease (PD), and the Pink1/Parkin pathway regulates mitochondrial integrity and function.An important question is whether the proteins encoded by these genes function to regulate activities of other cellular compartments. A study in mice,reported by Xiong et al. in this issue of the JCI, demonstrates that Pink1,Parkin, and DJ-1 can form a complex in the cytoplasm, with Pink1 and DJ-1 promoting the E3 ubiquitin ligase activity of Parkin to degrade substrates via the proteasome. This protein complex in the cytosol may or may not be related to the role of these proteins in regulating mitochondrial function or oxidative stress in vivo.
Figures
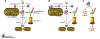
Comment on
-
Parkin, PINK1, and DJ-1 form a ubiquitin E3 ligase complex promoting unfolded protein degradation.J Clin Invest. 2009 Mar;119(3):650-60. doi: 10.1172/JCI37617. Epub 2009 Feb 23. J Clin Invest. 2009. PMID: 19229105 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

