Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins
- PMID: 19306879
- PMCID: PMC2801759
- DOI: 10.1016/j.febslet.2009.03.037
Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins
Abstract
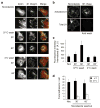
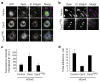
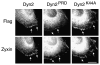
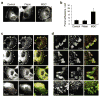
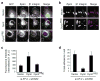
Cell migration requires the controlled disassembly of focal adhesions, but the underlying mechanisms remain poorly understood. Here, we show that adhesion turnover is mediated through dynamin- and clathrin-dependent endocytosis of activated beta1 integrins. Consistent with this, clathrin and the clathrin adaptors AP-2 and disabled-2 (DAB2) distribute along with dynamin 2 to adhesion sites prior to adhesion disassembly. Moreover, knockdown of either dynamin 2 or both clathrin adaptors blocks beta1 integrin internalization, leading to impaired focal adhesion disassembly and cell migration. Together, these results provide important insight into the mechanisms underlying adhesion disassembly and identify novel components of the disassembly pathway.
Figures
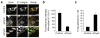

References
-
- Webb DJ, Parsons JT, Horwitz AF. Adhesion assembly, disassembly and turnover in migrating cells—over and over and over again. Nat Cell Biol. 2002;4:E97–E100. - PubMed
-
- Broussard JA, Webb DJ, Kaverina I. Asymmetric focal adhesion disassembly in motile cells. Curr Opin Cell Biol. 2008;20:85–90. - PubMed
-
- Webb DJ, Donais K, Whitmore LA, Thomas SM, Turner CE, Parsons JT, Horwitz AF. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell Biol. 2004;6:154–161. - PubMed
-
- Franco SJ, Rodgers MA, Perrin BJ, Han J, Bennin DA, Critchley DR, Huttenlocher A. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–983. - PubMed
-
- Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

