Satratoxin G interaction with 40S and 60S ribosomal subunits precedes apoptosis in the macrophage
- PMID: 19306889
- PMCID: PMC3124149
- DOI: 10.1016/j.taap.2009.03.006
Satratoxin G interaction with 40S and 60S ribosomal subunits precedes apoptosis in the macrophage
Abstract
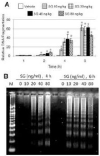
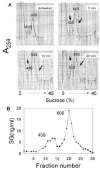
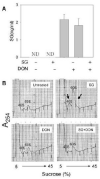
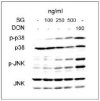
Satratoxin G (SG) and other macrocyclic trichothecene mycotoxins are potent inhibitors of eukaryotic translation that are potentially immunosuppressive. The purpose of this research was to test the hypothesis that SG-induced apoptosis in the macrophage correlates with binding of this toxin to the ribosome. Exposure of RAW 264.7 murine macrophages to SG at concentrations of 10 to 80 ng/ml induced DNA fragmentation within 4 h that was indicative of apoptosis. To relate these findings to ribosome binding of SG, RAW cells were exposed to different toxin concentrations for various time intervals, ribosomal fractions isolated by sucrose density gradient ultracentrifugation and resultant fractions analyzed for SG by competitive ELISA. SG was found to specifically interact with 40S and 60S ribosomal subunits as early as 5 min and that, at high concentrations or extended incubation times, the toxin induced polysome disaggregation. While co-incubation with the simple Type B trichothecene DON had no effect on SG uptake into cell cytoplasm, it inhibited SG binding to the ribosome, suggesting that the two toxins bound to identical sites and that SG binding was reversible. Although both SG and DON induced mobilization of p38 and JNK 1/2 to the ribosome, phosphorylation of ribosomal bound MAPKs occurred only after DON treatment. SG association with the 40S and 60S subunits was also observed in the PC-12 neuronal cell model which is similarly susceptible to apoptosis. To summarize, SG rapidly binds small and large ribosomal subunits in a concentration- and time-dependent manner that was consistent with induction of apoptosis.
Figures





References
-
- Bamburg JR. Biological and biochemical actions of trichothecene mycotoxins. Prog. Mol. Subcell. Biol. 1983;8:41–110.
-
- Chung YJ, Jarvis BB, Tak H, Pestka JJ. Immunochemical assay for satratoxin G and other macrocyclic trichothecenes associated with indoor air contamination by Stachybotrys chartarum. Toxicol. Mech. Meth. 2003a;13:247–252. - PubMed
-
- Chung YJ, Zhou HR, Pestka JJ. Transcriptional and posttranscriptional roles for p38 mitogen-activated protein kinase in upregulation of TNF-alpha expression by deoxynivalenol (vomitoxin) Toxicol. Appl. Pharmacol. 2003b;193:188–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

